Abstract
Exposure to endocrine disrupting chemicals (EDCs) is associated with dysfunctions of metabolism, energy balance, thyroid function and reproduction, and an increased risk of endocrine cancers. These multifactorial disorders can be 'programmed' through molecular epigenetic changes induced by exposure to EDCs early in life, the expression of which may not manifest until adulthood. In some cases, EDCs have detrimental effects on subsequent generations, which indicates that traits for disease predisposition may be passed to future generations by nongenomic inheritance. This Review discusses current understanding of the epigenetic mechanisms that underlie sexual differentiation of reproductive neuroendocrine systems in mammals and summarizes the literature on transgenerational epigenetic effects of representative EDCs: vinclozolin, diethylstilbesterol, bisphenol A and polychlorinated biphenyls. The article differentiates between context-dependent epigenetic transgenerational changes—namely, those that require environmental exposure, either via the EDC itself or through behavioral or physiological differences in parents—and germline-dependent epigenetic mechanisms. These processes, albeit discrete, are not mutually exclusive and can involve similar molecular mechanisms including DNA methylation and histone modifications and may predispose exposed individuals to transgenerational disruption of reproductive processes. New insights stress the crucial need to develop a clear understanding of how EDCs may program the epigenome of exposed individuals and their descendants.
Key Points
-
The hypothalamic neuroendocrine systems develop in a sexually dimorphic manner, largely because of differences in levels of gonadal steroids
-
Environmental endocrine disrupting chemicals (EDCs) impair the function of the neuroendocrine systems that control reproduction
-
Developmental exposure to EDCs, particularly during embryonic and early postnatal periods, permanently impairs functions and predisposes individuals to disease later in life owing to altered epigenetic programming
-
The mechanisms of EDC action include effects on the epigenetic molecular machinery that controls gene expression in hypothalamic and reproductive tissues
-
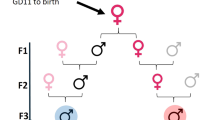
Effects of EDCs may be transmitted transgenerationally through molecular changes to the germline or through context-dependent modifications to somatic cells by continued exposures to EDCs or the individual's social or environmental context
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Weybridge, UK, Report No. EUR 17549, Environment and Climate Research Programme, DG XXI (European Commission, Brussels, 1996).
Kavlock, R. J. & Ankley, G. T. A perspective on the risk assessment process for endocrine-disruptive effects on wildlife and human health. Risk Anal. 16, 731–739 (1996).
Diamanti-Kandarakis, E. et al. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr. Rev. 30, 293–342 (2009).
Koistinen, J., Stenman, O., Haahti, H., Suonperä, M. & Paasivirta, J. Polychlorinated diphenyl ethers, dibenzo-p-dioxins, dibenzofurans and biphenyls in seals and sediment from the Gulf of Finland. Chemosphere 35, 1249–1269 (1997).
Barker, D. J., Eriksson, J. G., Forsén, T. & Osmond, C. Fetal origins of adult disease: strength of effects and biological basis. Int. J. Epidemiol. 31, 1235–1239 (2002).
Gore, A. C. Developmental programming and endocrine disruptor effects on reproductive neuroendocrine systems. Front. Neuroendocrinol. 29, 358–374 (2008).
Crews, D. et al. Transgenerational epigenetic imprints on mate preference. Proc. Natl Acad. Sci. USA 104, 5942–5946 (2007).
Crews, D., Willingham, E. & Skipper, J. K. Endocrine disruptors: present issues, future directions. Q. Rev. Biol. 75, 243–260 (2000).
Wingfield, J. C. Comparative endocrinology, environment and global change. Gen. Comp. Endocrinol. 157, 207–216 (2008).
Crews, D. Epigenetics and its implications for behavioral neuroendocrinology. Front. Neuroendocrinol. 29, 344–357 (2008).
Cornil, C. A., Ball, G. F. & Balthazart, J. Functional significance of the rapid regulation of brain estrogen action: where do the estrogens come from? Brain Res. 1126, 2–26 (2006).
Juntti, S. A. et al. The androgen receptor governs the execution, but not programming, of male sexual and territorial behaviors. Neuron 66, 260–272 (2010).
Lephart, E. D. et al. Brain aromatase cytochrome P-450 messenger RNA levels and enzyme activity during prenatal and perinatal development in the rat. Brain Res. Mol. Brain Res. 16, 187–192 (1992).
Bakker, J. et al. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat. Neurosci. 9, 220–226 (2006).
Davis, E. C., Popper, P. & Gorski, R. A. The role of apoptosis in sexual differentiation of the rat sexually dimorphic nucleus of the preoptic area. Brain Res. 734, 10–18 (1996).
Davis, E. C., Shryne, J. E. & Gorski, R. A. A revised critical period for the sexual differentiation of the sexually dimorphic nucleus of the preoptic area in the rat. Neuroendocrinology 62, 579–585 (1995).
Davis, E. C., Shryne, J. E. & Gorski, R. A. Structural sexual dimorphisms in the anteroventral periventricular nucleus of the rat hypothalamus are sensitive to gonadal steroids perinatally, but develop peripubertally. Neuroendocrinology 63, 142–148 (1996).
Phoenix, C. H., Goy, R. W., Gerall, A. A. & Young, W. C. Organizing action of prenatally administered testosterone propionate on the tissues mediating mating behavior in the female guinea pig. Endocrinology 65, 369–382 (1959).
Baccarelli, A. & Bollati, V. Epigenetics and environmental chemicals. Curr. Opin. Pediatr. 21, 243–251 (2009).
Crews, D. Epigenetic modifications of brain and behavior: theory and practice. Horm. Behav. doi:10.1016/j.yhbeh.2010.07.001
Bolander, F. F. Molecular Endocrinology 3rd edn (Elsevier Academic Press, San Diego, 2004).
McCarthy, M. M. et al. The epigenetics of sex differences in the brain. J. Neurosci. 29, 12815–12823 (2009).
Westberry, J. M., Trout, A. L. & Wilson, M. E. Epigenetic regulation of estrogen receptor alpha gene expression in the mouse cortex during early postnatal development. Endocrinology 151, 731–740 (2010).
Kurian, J. R., Olesen, K. M. & Auger, A. P. Sex differences in epigenetic regulation of the estrogen receptor-alpha promoter within the developing preoptic area. Endocrinology 151, 2297–2305 (2010).
Tsai, H. W., Grant, P. A. & Rissman, E. F. Sex differences in histone modifications in the neonatal mouse brain. Epigenetics 4, 47–53 (2009).
Murray, E. K., Hien, A., de Vries, G. J. & Forger, N. G. Epigenetic control of sexual differentiation of the bed nucleus of the stria terminalis. Endocrinology 150, 4241–4247 (2009).
Champagne, F. A. Epigenetic mechanisms and the transgenerational effects of maternal care. Front. Neuroendocrinol. 29, 386–397 (2008).
Cameron, N. M. et al. Epigenetic programming of phenotypic variations in reproductive strategies in the rat through maternal care. J. Neuroendocrinol. 20, 795–801 (2008).
Homburg, R. Androgen circle of polycystic ovary syndrome. Hum. Reprod. 24, 1548–1555 (2009).
Reik, W., Dean, W. & Walter, J. Epigenetic reprogramming in mammalian development. Science 293, 1089–1093 (2001).
Jablonka, E. & Raz, G. Transgenerational epigenetic inheritance: prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 84, 131–176 (2009).
Gray, L. E. et al. Effects of environmental antiandrogens on reproductive development in experimental animals. Hum. Reprod. Update 7, 248–264 (2001).
Anway, M. D., Cupp, A. S., Uzumcu, M. & Skinner, M. K. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science 308, 1466–1469 (2005).
Stouder, C. & Paoloni-Giacobino, A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction 139, 373–379 (2010).
Anway, M. D., Memon, M. A., Uzumcu, M. & Skinner, M. K. Transgenerational effect of the endocrine disruptor vinclozolin on male spermatogenesis. J. Androl. 27, 868–879 (2006).
Anway, M. D., Rekow, S. S. & Skinner, M. K. Transgenerational epigenetic programming of the embryonic testis transcriptome. Genomics 91, 30–40 (2008).
Anway, M. D., Rekow, S. S. & Skinner, M. K. Comparative anti-androgenic actions of vinclozolin and flutamide on transgenerational adult onset disease and spermatogenesis. Reprod. Toxicol. 26, 100–106 (2008).
Anway, M. D., Leathers, C. & Skinner, M. K. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology 147, 5515–5523 (2006).
Nilsson, E. E., Anway, M. D., Stanfield, J. & Skinner, M. K. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction 135, 713–721 (2008).
Inawaka, K. et al. Maternal exposure to anti-androgenic compounds, vinclozolin, flutamide and procymidone, has no effects on spermatogenesis and DNA methylation in male rats of subsequent generations. Toxicol. Appl. Pharmacol. 237, 178–187 (2009).
Schneider, S., Kaufmann, W., Buesen, R. & van Ravenzwaay, B. Vinclozolin—the lack of a transgenerational effect after oral maternal exposure during organogenesis. Reprod. Toxicol. 25, 352–360 (2008).
Gore, A. C. Neuroendocrine targets of endocrine disruptors. Hormones (Athens) 9, 16–27 (2010).
Skinner, M. K., Anway, M. D., Savenkova, M. I., Gore, A. C. & Crews, D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE 3, e3745 (2008).
Herbst, A. L. The current status of the DES-exposed population. Obstet. Gynecol. Annu. 10, 267–278 (1981).
Herbst, A. L., Ulfelder, H. & Poskanzer, D. C. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N. Engl. J. Med. 284, 878–881 (1971).
McLachlan, J. A. & Newbold, R. R. Estrogens and development. Environ. Health Perspect. 75, 25–27 (1987).
Titus-Ernstoff, L. et al. Birth defects in the sons and daughters of women who were exposed in utero to diethylstilbestrol (DES). Int. J. Androl. 33, 377–384 (2010).
Titus-Ernstoff, L. et al. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES). Int. J. Epidemiol. 35, 862–868 (2006).
Klip, H., Burger, C. W., de Kraker, J. & van Leeuwen, F. E. Risk of cancer in the offspring of women who underwent ovarian stimulation for IVF. Hum. Reprod. 16, 2451–2458 (2001).
Blatt, J., Van Le, L., Weiner, T. & Sailer, S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J. Pediatr. Hematol. Oncol. 25, 635–636 (2003).
Newbold, R. R. et al. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis 21, 1355–1363 (2000).
Newbold, R. R. et al. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis 19, 1655–1663 (1998).
Walker, B. E. & Haven, M. I. Intensity of multigenerational carcinogenesis from diethylstilbestrol in mice. Carcinogenesis 18, 791–793 (1997).
Li, S. et al. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 57, 4356–4359 (1997).
Newbold, R. R., Padilla-Banks, E. & Jefferson, W. N. Adverse effects of the model environmental estrogen diethylstilbestrol are transmitted to subsequent generations. Endocrinology 147 (Suppl.), S11–S17 (2006).
Li, S. et al. Promoter CpG methylation of Hox-a10 and Hox-a11 in mouse uterus not altered upon neonatal diethylstilbestrol exposure. Mol. Carcinog. 32, 213–219 (2001).
Bromer, J. G., Wu, J., Zhou, Y. & Taylor, H. S. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology 150, 3376–3382 (2009).
Sato, K. et al. Neonatal exposure to diethylstilbestrol alters the expression of DNA methyltransferases and methylation of genomic DNA in the epididymis of mice. Endocr. J. 53, 331–337 (2006).
Sato, K. et al. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr. J. 56, 131–139 (2009).
Bredfeldt, T. G. et al. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol. Endocrinol. 24, 993–1006 (2010).
Greathouse, K. L. et al. Identification of uterine leiomyoma genes developmentally reprogrammed by neonatal exposure to diethylstilbestrol. Reprod. Sci. 15, 765–778 (2008).
Pearce, E. N. & Braverman, L. E. Environmental pollutants and the thyroid. Best Pract. Res. Clin. Endocrinol. Metab. 23, 801–813 (2009).
Vandenberg, L. N., Maffini, M. V., Sonnenschein, C., Rubin, B. S. & Soto, A. M. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr. Rev. 30, 75–95 (2009).
Salian, S., Doshi, T. & Vanage, G. Perinatal exposure of rats to bisphenol A affects the fertility of male offspring. Life Sci. 85, 742–752 (2009).
Salian, S., Doshi, T. & Vanage, G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to bisphenol A. Life Sci. 85, 11–18 (2009).
Ho, S. M., Tang, W. Y., Belmonte de Frausto, J. & Prins, G. S. Developmental exposure to estradiol and bisphenol A increases susceptibility to prostate carcinogenesis and epigenetically regulates phosphodiesterase type 4 variant 4. Cancer Res. 66, 5624–5632 (2006).
Yaoi, T. et al. Genome-wide analysis of epigenomic alterations in fetal mouse forebrain after exposure to low doses of bisphenol A. Biochem. Biophys. Res. Commun. 376, 563–567 (2008).
Dolinoy, D. C., Huang, D. & Jirtle, R. L. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc. Natl Acad. Sci. USA 104, 13056–13061 (2007).
Rosenfeld, C. S. Animal models to study environmental epigenetics. Biol. Reprod. 82, 473–488 (2010).
Palanza, P. L., Howdeshell, K. L., Parmigiani, S. & vom Saal, F. S. Exposure to a low dose of bisphenol A during fetal life or in adulthood alters maternal behavior in mice. Environ. Health Perspect. 110 (Suppl. 3), 415–422 (2002).
Chung, Y. W., Nunez, A. A. & Clemens, L. G. Effects of neonatal polychlorinated biphenyl exposure on female sexual behavior. Physiol. Behav. 74, 363–370 (2001).
Salama, J., Chakraborty, T. R., Ng, L. & Gore, A. C. Effects of polychlorinated biphenyls on estrogen receptor-beta expression in the anteroventral periventricular nucleus. Environ. Health Perspect. 111, 1278–1282 (2003).
Steinberg, R. M., Juenger, T. E. & Gore, A. C. The effects of prenatal PCBs on adult female paced mating reproductive behaviors in rats. Horm. Behav. 51, 364–372 (2007).
Steinberg, R. M., Walker, D. M., Juenger, T. E., Woller, M. J. & Gore, A. C. Effects of perinatal polychlorinated biphenyls on adult female rat reproduction: development, reproductive physiology, and second generational effects. Biol. Reprod. 78, 1091–1101 (2008).
Desaulniers, D. et al. Effects of postnatal exposure to a mixture of polychlorinated biphenyls, p,p'-dichlorodiphenyltrichloroethane, and p-p'-dichlorodiphenyldichloroethene in prepubertal and adult female Sprague-Dawley rats. Int. J. Toxicol. 24, 111–127 (2005).
Desaulniers, D. et al. Effects of mixtures of polychlorinated biphenyls, methylmercury, and organochlorine pesticides on hepatic DNA methylation in prepubertal female Sprague-Dawley rats. Int. J. Toxicol. 28, 294–307 (2009).
Shimada, M. et al. Gene expression profiles in the brain of the neonate mouse perinatally exposed to methylmercury and/or polychlorinated biphenyls. Arch. Toxicol. 84, 271–286 (2010).
Walker, D. M. & Gore, A. C. in Endocrine-Disrupting Chemicals: From Basic Research to Clinical Practice Ch. 4 (ed. Gore, A. C.) 63–109 (Humana Press, New Jersey, 2007).
Qiu, J. Epigenetics: unfinished symphony. Nature 441, 143–145 (2006).
Kavlock, R. J. et al. Research needs for the risk assessment of health and environmental effects of endocrine disruptors: a report of the U.S. EPA-sponsored workshop. Environ. Health Perspect. 104 (Suppl. 4), 715–740 (1996).
Bowler, P. J. The changing meaning of “evolution”. J. Hist. Ideas 36, 95–114 (1975).
Gorski, R. A., Harlan, R. E., Jacobson, C. D., Shryne, J. E. & Southam, A. M. Evidence for the existence of a sexually dimorphic nucleus in the preoptic area of the rat. J. Comp. Neurol. 193, 529–539 (1980).
Simerly, R. B., Swanson, L. W. & Gorski, R. A. The distribution of monoaminergic cells and fibers in a periventricular preoptic nucleus involved in the control of gonadotropin release: immunohistochemical evidence for a dopaminergic sexual dimorphism. Brain Res. 330, 55–64 (1985).
Ryan, B. C., Hotchkiss, A. K., Crofton, K. M. & Gray, L. E. Jr. In utero and lactational exposure to bisphenol A, in contrast to ethinyl estradiol, does not alter sexually dimorphic behavior, puberty, fertility, and anatomy of female LE rats. Toxicol. Sci. 114, 133–148 (2010).
Myers, J. P. et al. Why public health agencies cannot depend on good laboratory practices as a criterion for selecting data: the case of bisphenol A. Environ. Health Perspect. 117, 309–315 (2009).
Acknowledgements
We are grateful to David Crews for helpful discussions and comments on this manuscript. Belinda Lehmkuhle provided expert assistance with the figures. Work described in this article was supported by NIH 1RC1 ES018139.
Author information
Authors and Affiliations
Contributions
Both authors contributed to researching data for the article, a substantial contribution to discussion of the content, and to writing the article and reviewing/editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Walker, D., Gore, A. Transgenerational neuroendocrine disruption of reproduction. Nat Rev Endocrinol 7, 197–207 (2011). https://doi.org/10.1038/nrendo.2010.215
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2010.215
This article is cited by
-
Follicle-stimulating hormone (FSH) promotes retinol uptake and metabolism in the mouse ovary
Reproductive Biology and Endocrinology (2018)
-
Transgenerational effects of polychlorinated biphenyls: 1. Development and physiology across 3 generations of rats
Environmental Health (2018)
-
Analgesic exposure in pregnant rats affects fetal germ cell development with inter-generational reproductive consequences
Scientific Reports (2016)
-
Transgenerational Inheritance of Paternal Neurobehavioral Phenotypes: Stress, Addiction, Ageing and Metabolism
Molecular Neurobiology (2016)