Abstract
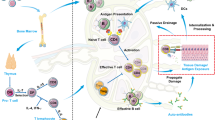
Type 1 diabetes mellitus (T1DM) is a prototypic organ-specific autoimmune disease that results from selective destruction of insulin-secreting β-cells by immune-mediated inflammation (insulitis), that is, the infiltration of pancreatic islets by autoreactive CD4+ and CD8+ T lymphocytes. Current treatment is substitutive—chronic use of exogenous insulin—which, in spite of considerable advances, is still associated with constraints and lack of effectiveness over the long-term in relation to the prevention of vascular and neurological complications. Finding a cure for T1DM is an important medical health challenge, as the disease's incidence is steadily increasing in industrialized countries and projections of future prevalence are alarming. Crucially, as T1DM mainly affects children and young adults, any candidate immune therapy must be safe and avoid chronic use of immunosuppressants that promote sustained depression of immune responses. The ideal approach would, therefore, involve induction or, in the case of established T1DM, restoration of immune tolerance to target autoantigens. This Review presents, in particular, two strategies that are still in clinical development but hold great promise. These strategies are focused on the use of candidate autoantigens and anti-CD3 monoclonal antibodies.
Key Points
-
Type 1 diabetes mellitus (T1DM) is a prototypic autoimmune disease whose pathophysiology involves autoantigen-presenting cells (dendritic cells, macrophages and B lymphocytes), T lymphocytes and target insulin-secreting β cells
-
Immune destruction of β cells in humans starts months to years before the advent of overt hyperglycemia
-
The incidence of T1DM has steadily increased in developed countries over the past few decades; prevalence in children <15 years is predicted to increase by 70% between 2005 and 2020
-
Given the autoimmune origin of T1DM, immune intervention is the only option to find a real cure
-
The possibility exists to reverse T1DM once hyperglycemia is established, as β cells have not all been destroyed
-
Chronic immunosuppression may be effective, but is not the way to go because of the potential long-term adverse effects
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Bach, J. F. Insulin-dependent diabetes mellitus as an autoimmune disease. Endocrine Rev. 15, 516–542 (1994).
Bach, J. F. The effect of infections on susceptibility to autoimmune and allergic diseases. N. Engl. J. Med. 347, 911–920 (2002).
Bottazzo, G. F., Florin-Christensen, A. & Doniach, D. Islet-cell antibodies in diabetes mellitus with autoimmune polyendocrine deficiencies. Lancet 2, 1279–1283 (1974).
Srikanta, S., Ganda, O. P., Eisenbarth, G. S. & Soeldner, J. S. Islet-cell antibodies and beta-cell function in monozygotic triplets and twins initially discordant for type 1 diabetes mellitus. N. Engl. J. Med. 308, 322–325 (1983).
Bingley, P. J. & Gale, E. A. Progression to type 1 diabetes in islet cell antibody-positive relatives in the European Nicotinamide Diabetes Intervention Trial: the role of additional immune, genetic and metabolic markers of risk. Diabetologia 49, 881–890 (2006).
Makino, S. et al. Breeding of a non-obese, diabetic strain of mice. Exp. Anim. 29, 1–13 (1980).
Crisá, L., Mordes, J. P. & Rossini, A. A. Autoimmune diabetes mellitus in the BB rat. Diabetes Metab. Rev. 8, 4–37 (1992).
Katz, J. D., Wang, B., Haskins, K., Benoist, C. & Mathis, D. Following a diabetogenic T cell from genesis through pathogenesis. Cell 74, 1089–1100 (1993).
Bach, J. F. & Chatenoud, L. Tolerance to islet autoantigens in type 1 diabetes. Annu. Rev. Immunol. 19, 131–161 (2001).
Boitard, C., Yasunami, R., Dardenne, M. & Bach, J. F. T cell-mediated inhibition of the transfer of autoimmune diabetes in NOD mice. J. Exp. Med. 169, 1669–1680 (1989).
Chatenoud, L., Salomon, B. & Bluestone, J. A. Suppressor T cells--they're back and critical for regulation of autoimmunity! Immunol. Rev. 182, 149–163 (2001).
Salomon, B. et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity 12, 431–440 (2000).
Bach, J. F. Regulatory T cells under scrutiny. Nat. Rev. Immunol. 3, 189–198 (2003).
Eizirik, D. L., Colli, M. L. & Ortis, F. The role of inflammation in insulitis and beta-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 5, 219–226 (2009).
Nakayama, M. et al. Prime role for an insulin epitope in the development of type 1 diabetes in NOD mice. Nature 435, 220–223 (2005).
Baekkeskov, S. et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347, 151–156 (1990).
Lan, M. S., Wasserfall, C., Maclaren, N. K. & Notkins, A. L. IA-2, a transmembrane protein of the protein tyrosine phosphatase family, is a major autoantigen in insulin-dependent diabetes mellitus. Proc. Natl Acad. Sci. USA 93, 6367–6370 (1996).
Elias, D., Marcus, H., Reshef, T., Ablamunits, V. & Cohen, I. R. Induction of diabetes in standard mice by immunization with the p277 peptide of a 60-kDa heat shock protein. Eur. J. Immunol. 25, 2851–2857 (1995).
Lieberman, S. M. et al. Identification of the beta cell antigen targeted by a prevalent population of pathogenic CD8+ T cells in autoimmune diabetes. Proc. Natl Acad. Sci. USA 100, 8384–8388 (2003).
Wenzlau, J. M. et al. SlC30A8 is a major target of humoral autoimmunity in type 1 diabetes and a predictive marker in prediabetes. Ann. NY Acad. Sci. 1150, 256–259 (2008).
Harjutsalo, V., Sjöberg, L. & Tuomilehto, J. Time trends in the incidence of type 1 diabetes in Finnish children: a cohort study. Lancet 371, 1777–1782 (2008).
Patterson, C. C., Dahlquist, G. G., Gyürüs, E., Green, A. & Soltész, G. Incidence trends for childhood type 1 diabetes in Europe during 1989–2003 and predicted new cases 2005–20: a multicentre prospective registration study. Lancet 373, 2027–2033 (2009).
Stiller, C. R. et al. Effects of cyclosporine immunosuppression in insulin-dependent diabetes mellitus of recent onset. Science 223, 1362–1367 (1984).
Feutren, G. et al. Cyclosporin increases the rate and length of remissions in insulin-dependent diabetes of recent onset. Results of a multicentre double-blind trial. Lancet 2, 119–124 (1986).
Silverstein, J. et al. Immunosuppression with azathioprine and prednisone in recent-onset insulin-dependent diabetes mellitus. N. Engl. J. Med. 319, 599–604 (1988).
Strandell, E., Eizirik, D. L. & Sandler, S. Reversal of beta-cell suppression in vitro in pancreatic islets isolated from nonobese diabetic mice during the phase preceding insulin-dependent diabetes mellitus. J. Clin. Invest. 85, 1944–1950 (1990).
Sreenan, S. et al. Increased beta-cell proliferation and reduced mass before diabetes onset in the nonobese diabetic mouse. Diabetes 48, 989–996 (1999).
Silverman, G. J. & Weisman, S. Rituximab therapy and autoimmune disorders: prospects for anti-B cell therapy. Arthritis Rheum. 48, 1484–1492 (2003).
Leandro, M. J., Cambridge, G., Edwards, J. C., Ehrenstein, M. R. & Isenberg, D. A. B-cell depletion in the treatment of patients with systemic lupus erythematosus: a longitudinal analysis of 24 patients. Rheumatology (Oxford) 44, 1542–1545 (2005).
Emery, P. et al. The efficacy and safety of rituximab in patients with active rheumatoid arthritis despite methotrexate treatment: results of a phase IIB randomized, double-blind, placebo-controlled, dose-ranging trial. Arthritis Rheum. 54, 1390–1400 (2006).
Serreze, D. V. et al. B lymphocytes are essential for the initiation of T cell-mediated autoimmune diabetes: analysis of a new “speed congenic” stock of NOD.Ig mu(null) mice. J. Exp. Med. 184, 2049–2053 (1996).
Hu, C. Y. et al. Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J. Clin. Invest. 117, 3857–3867 (2007).
Xiu, Y. et al. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J. Immunol. 180, 2863–2875 (2008).
Hauser, S. L. et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N. Engl. J. Med. 358, 676–688 (2008).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of beta-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
Dresser, D. W. Specific inhibition of antibody production. II. Paralysis induced in adult mice by small quantities of protein antigen. Immunology 5, 378–388 (1962).
Dresser, D. W. & Mitchison, N. A. The mechanism of immunological paralysis. Adv. Immunol. 8, 129–181 (1968).
Kaufman, D. L. et al. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 366, 69–72 (1993).
Tisch, R. et al. Immune response to glutamic acid decarboxylase correlates with insulitis in non-obese diabetic mice. Nature 366, 72–75 (1993).
Elliott, J. F. et al. Immunization with the larger isoform of mouse glutamic acid decarboxylase (GAD67) prevents autoimmune diabetes in NOD mice. Diabetes 43, 1494–1499 (1994).
Li, A. F. & Escher, A. Intradermal or oral delivery of GAD-encoding genetic vaccines suppresses type 1 diabetes. DNA Cell Biol. 22, 227–232 (2003).
Goudy, K. S., Wang, B. & Tisch, R. Gene gun-mediated DNA vaccination enhances antigen-specific immunotherapy at a late preclinical stage of type 1 diabetes in nonobese diabetic mice. Clin. Immunol. 129, 49–57 (2008).
Tisch, R. et al. Antigen-specific mediated suppression of beta cell autoimmunity by plasmid DNA vaccination. J. Immunol. 166, 2122–2132 (2001).
Tian, J. et al. Nasal administration of glutamate decarboxylase (GAD65) peptides induces Th2 responses and prevents murine insulin-dependent diabetes. J. Exp. Med. 183, 1561–1567 (1996).
Olcott, A. P. et al. Antigen-based therapies using ignored determinants of beta cell antigens can more effectively inhibit late-stage autoimmune disease in diabetes-prone mice. J. Immunol. 175, 1991–1999 (2005).
Atkinson, M. A., Maclaren, N. K. & Luchetta, R. Insulitis and diabetes in NOD mice reduced by prophylactic insulin therapy. Diabetes 39, 933–937 (1990).
Daniel, D. & Wegmann, D. R. Protection of nonobese diabetic mice from diabetes by intranasal or subcutaneous administration of insulin peptide B-(9–23). Proc. Natl Acad. Sci. USA 93, 956–960 (1996).
Zhang, Z. J., Davidson, L., Eisenbarth, G. & Weiner, H. L. Suppression of diabetes in nonobese diabetic mice by oral administration of porcine insulin. Proc. Natl Acad. Sci. USA 88, 10252–10256 (1991).
Bergerot, I. et al. A cholera toxoid-insulin conjugate as an oral vaccine against spontaneous autoimmune diabetes. Proc. Natl Acad. Sci. USA 94, 4610–4614 (1997).
Harrison, L. C., Dempsey-Collier, M., Kramer, D. R. & Takahashi, K. Aerosol insulin induces regulatory CD8 gamma delta T cells that prevent murine insulin-dependent diabetes. J. Exp. Med. 184, 2167–2174 (1996).
Karounos, D. G., Bryson, J. S. & Cohen, D. A. Metabolically inactive insulin analog prevents type I diabetes in prediabetic NOD mice. J. Clin. Invest. 100, 1344–1348 (1997).
Elias, D. & Cohen, I. R. Peptide therapy for diabetes in NOD mice. Lancet 343, 704–706 (1994).
Chaillous, L. et al. Oral insulin administration and residual beta-cell function in recent-onset type 1 diabetes: a multicentre randomised controlled trial. Diabète Insuline Orale group. Lancet 356, 545–549 (2000).
Pozzilli, P. et al. No effect of oral insulin on residual beta-cell function in recent-onset type I diabetes (the IMDIAB VII). IMDIAB Group. Diabetologia 43, 1000–1004 (2000).
Walter, M., Philotheou, A., Bonnici, F., Ziegler, A. G. & Jimenez, R. No Effect of the altered-peptide ligand NBI-6024 on beta cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care 32, 2036–2040 (2009).
Raz, I. et al. Beta-cell function in new-onset type 1 diabetes and immunomodulation with a heat-shock protein peptide (DiaPep277): a randomised, double-blind, phase II trial. Lancet 358, 1749–1753 (2001).
Skyler, J. & Diabetes Prevention Trial-Type 1 Diabetes Study Group. Effects of insulin in relatives of patients with type 1 diabetes mellitus. N. Engl. J. Med. 346, 1685–1691 (2002).
Näntö-Salonen, K. et al. Nasal insulin to prevent type 1 diabetes in children with HLA genotypes and autoantibodies conferring increased risk of disease: a double-blind, randomised controlled trial. Lancet 372, 1746–1755 (2008).
Ludvigsson, J. et al. GAD treatment and insulin secretion in recent-onset type 1 diabetes. N. Engl. J. Med. 359, 1909–1920 (2008).
Monaco, A. P., Wood, M. L. & Russell, P. S. Studies on heterologous antilymphocyte serum in mice. III. Immunological tolerance and chimerism produced across the H2-locus with adult thymectomy and antilymphocyte serum. Ann. NY Acad. Sci. 129, 190–209 (1966).
Plain, K. M., Chen, J., Merten, S., He, X. Y. & Hall, B. M. Induction of specific tolerance to allografts in rats by therapy with non-mitogenic, non-depleting anti-CD3 monoclonal antibody: association with TH2 cytokines not anergy. Transplantation 67, 605–613 (1999).
Cobbold, S. P., Qin, S., Leong, L. Y., Martin, G. & Waldmann, H. Reprogramming the immune system for peripheral tolerance with CD4 and CD8 monoclonal antibodies. Immunol. Rev. 129, 165–201 (1992).
Quezada, S. A., Jarvinen, L. Z., Lind, E. F. & Noelle, R. J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 (2004).
Waldmann, H. & Cobbold, S. Regulating the immune response to transplants. a role for CD4+ regulatory cells? Immunity 14, 399–406 (2001).
Chatenoud, L., Thervet, E., Primo, J. & Bach, J. F. Anti-CD3 antibody induces long-term remission of overt autoimmunity in nonobese diabetic mice. Proc. Natl Acad. Sci. USA 91, 123–127 (1994).
Chatenoud, L., Primo, J. & Bach, J. F. CD3 antibody-induced dominant self tolerance in overtly diabetic NOD mice. J. Immunol. 158, 2947–2954 (1997).
Xu, D. et al. In vitro characterization of five humanized OKT3 effector function variant antibodies. Cell. Immunol. 200, 16–26 (2000).
Herold, K. C. et al. Anti-CD3 monoclonal antibody in new-onset type 1 diabetes mellitus. N. Engl. J. Med. 346, 1692–1698 (2002).
Herold, K. C. et al. A single course of anti-CD3 monoclonal antibody hOKT3gamma1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes 54, 1763–1769 (2005).
Bolt, S. et al. The generation of a humanized, non-mitogenic CD3 monoclonal antibody which retains in vitro immunosuppressive properties. Eur. J. Immunol. 23, 403–411 (1993).
Keymeulen, B. et al. Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N. Engl. J. Med. 352, 2598–2608 (2005).
Keymeulen, B. et al. Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia (in press).
Keymeulen, B. et al. Transient Epstein Barr Virus reactivation in CD3 monoclonal antibody-treated patients. Blood doi:10.1182/blood-2009-02-204875.
Lipsky, P. E. et al. Infliximab and methotrexate in the treatment of rheumatoid arthritis. Anti-Tumor Necrosis Factor Trial in Rheumatoid Arthritis with Concomitant Therapy Study Group. N. Engl. J. Med. 343, 1594–1602 (2000).
Reich, K. et al. Infliximab induction and maintenance therapy for moderate-to-severe psoriasis: a phase III, multicentre, double-blind trial. Lancet 366, 1367–1374 (2005).
Present, D. H. et al. Infliximab for the treatment of fistulas in patients with Crohn's disease. N. Engl. J. Med. 340, 1398–1405 (1999).
Mastrandrea, L. et al. Etanercept treatment in children with new-onset type 1 diabetes: pilot randomized, placebo-controlled, double-blind study. Diabetes Care 32, 1244–1249 (2009).
Miller, D. H. et al. A controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 348, 15–23 (2003).
Polman, C. H. et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354, 899–910 (2006).
Coles, A. J. et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N. Engl. J. Med. 359, 1786–1801 (2008).
Bielekova, B. et al. Encephalitogenic potential of the myelin basic protein peptide (amino acids 83–99) in multiple sclerosis: results of a phase II clinical trial with an altered peptide ligand. Nat. Med. 6, 1167–1175 (2000).
Bercovici, N. et al. Chronic intravenous injections of antigen induce and maintain tolerance in T cell receptor-transgenic mice. Eur. J. Immunol. 29, 345–354 (1999).
Muir, A. et al. Insulin immunization of nonobese diabetic mice induces a protective insulitis characterized by diminished intraislet interferon-gamma transcription. J. Clin. Invest. 95, 628–634 (1995).
Elias, D. & Cohen, I. R. Treatment of autoimmune diabetes and insulitis in NOD mice with heat shock protein 60 peptide p277. Diabetes 44, 1132–1138 (1995).
Elias, D. et al. Hsp60 peptide therapy of NOD mouse diabetes induces a Th2 cytokine burst and downregulates autoimmunity to various beta-cell antigens. Diabetes 46, 758–764 (1997).
Tian, J., Lehmann, P. V. & Kaufman, D. L. Determinant spreading of T helper cell 2 (Th2) responses to pancreatic islet autoantigens. J. Exp. Med. 186, 2039–2043 (1997).
Tian, J. D. et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes- prone mice. Nat. Med. 2, 1348–1353 (1996).
Tisch, R., Wang, B. & Serreze, D. V. Induction of glutamic acid decarboxylase 65-specific Th2 cells and suppression of autoimmune diabetes at late stages of disease is epitope dependent. J. Immunol. 163, 1178–1187 (1999).
Gaur, A. et al. Amelioration of relapsing experimental autoimmune encephalomyelitis with altered myelin basic protein peptides involves different cellular mechanisms. J. Neuroimmunol. 74, 149–158 (1997).
Hancock, W. W., Polanski, M., Zhang, J., Blogg, N. & Weiner, H. L. Suppression of insulitis in non-obese diabetic (NOD) mice by oral insulin administration is associated with selective expression of interleukin-4 and -10, transforming growth factor-beta, and prostaglandin-E. Am. J. Pathol. 147, 1193–1199 (1995).
Chatenoud, L. CD3-specific antibody-induced active tolerance: from bench to bedside. Nat. Rev. Immunol. 3, 123–132 (2003).
Chatenoud, L. & Bluestone, J. A. CD3-specific antibodies: a portal to the treatment of autoimmunity. Nat. Rev. Immunol. 7, 622–632 (2007).
You, S. et al. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes 54, 1415–1422 (2005).
You, S. et al. Adaptive TGF-beta-dependent regulatory T cells control autoimmune diabetes and are a privileged target of anti-CD3 antibody treatment. Proc. Natl Acad. Sci. USA 104, 6335–6340 (2007).
Kohm, A. P. et al. Treatment with nonmitogenic anti-CD3 monoclonal antibody induces CD4+ T cell unresponsiveness and functional reversal of established experimental autoimmune encephalomyelitis. J. Immunol. 174, 4525–4534 (2005).
Belghith, M. et al. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat. Med. 9, 1202–1208 (2003).
You, S., Thieblemont, N., Alyanakian, M. A., Bach, J. F. & Chatenoud, L. Transforming growth factor-beta and T-cell-mediated immunoregulation in the control of autoimmune diabetes. Immunol. Rev. 212, 185–202 (2006).
Perruche, S. et al. CD3-specific antibody-induced immune tolerance involves transforming growth factor-beta from phagocytes digesting apoptotic T cells. Nat. Med. 14, 528–535 (2008).
Li, M. O., Wan, Y. Y., Sanjabi, S., Robertson, A. K. & Flavell, R. A. Transforming growth factor-beta regulation of immune responses. Annu. Rev. Immunol. 24, 99–146 (2006).
Steinman, R. M., Hawiger, D. & Nussenzweig, M. C. Tolerogenic dendritic cells. Annu. Rev. Immunol. 21, 685–711 (2003).
Rutella, S., Danese, S. & Leone, G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood 108, 1435–1440 (2006).
Yamazaki, S. et al. Dendritic cells are specialized accessory cells along with TGF- for the differentiation of Foxp3+ CD4+ regulatory T cells from peripheral Foxp3 precursors. Blood 110, 4293–4302 (2007).
Ochi, H. et al. Oral CD3-specific antibody suppresses autoimmune encephalomyelitis by inducing CD4+CD25-LAP+ T cells. Nat. Med. 12, 627–635 (2006).
Woodle, E. S. et al. Phase I trial of a humanized, Fc receptor nonbinding OKT3 antibody, huOKT3gamma1(Ala-Ala) in the treatment of acute renal allograft rejection. Transplantation 68, 608–616 (1999).
Friend, P. J. et al. Phase I study of an engineered aglycosylated humanized CD3 antibody in renal transplant rejection. Transplantation 68, 1632–1637 (1999).
Ferran, C. et al. Reduction of morbidity and cytokine release in anti-CD3 MoAb-treated mice by corticosteroids. Transplantation 50, 642–648 (1990).
Ferran, C. et al. Cascade modulation by anti-tumor necrosis factor monoclonal antibody of interferon-gamma, interleukin 3 and interleukin 6 release after triggering of the CD3/T cell receptor activation pathway. Eur. J. Immunol. 21, 2349–2353 (1991).
Ferran, C. et al. Anti-tumor necrosis factor modulates anti-CD3-triggered T cell cytokine gene expression in vivo. J. Clin. Invest. 93, 2189–2196 (1994).
Charpentier, B. et al. Evidence that antihuman tumor necrosis factor monoclonal antibody prevents OKT3-induced acute syndrome. Transplantation 54, 997–1002 (1992).
Wajchenberg, B. L. Beta-cell failure in diabetes and preservation by clinical treatment. Endocr. Rev. 28, 187–218 (2007).
Sherry, N. A. et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology 148, 5136–5144 (2007).
Hale, G. et al. Pharmacokinetics and antibody responses to a CD3 antibody used in the treatment of type I diabetes. J. Clin. Pharmacol. (in press).
Bresson, D. et al. Anti-CD3 and nasal proinsulin combination therapy enhances remission from recent-onset autoimmune diabetes by inducing Tregs. J. Clin. Invest. 116, 1371–1381 (2006).
Lang, K. S. et al. Toll-like receptor engagement converts T-cell autoreactivity into overt autoimmune disease. Nat. Med. 11, 138–145 (2005).
Ohashi, P. S. et al. Ablation of “tolerance” and induction of diabetes by virus infection in viral antigen transgenic mice. Cell 65, 305–317 (1991).
Jenkins, M. K. & Schwartz, R. H. Antigen presentation by chemically modified splenocytes induces antigen-specific T cell unresponsiveness in vitro and in vivo. J. Exp. Med. 165, 302–319 (1987).
Bluestone, J. A. & Abbas, A. K. Natural versus adaptive regulatory T cells. Nat. Rev. Immunol. 3, 253–257 (2003).
Roncarolo, M. G. et al. Interleukin-10-secreting type 1 regulatory T cells in rodents and humans. Immunol. Rev. 212, 28–50 (2006).
Izcue, A., Coombes, J. L. & Powrie, F. Regulatory T cells suppress systemic and mucosal immune activation to control intestinal inflammation. Immunol. Rev. 212, 256–271 (2006).
Sakaguchi, S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Annu. Rev. Immunol. 22, 531–562 (2004).
Shevach, E. M. CD4+ CD25+ suppressor T cells: more questions than answers. Nat. Rev. Immunol. 2, 389–400 (2002).
Carpenter, P. A. et al. Non-Fc receptor-binding humanized anti-CD3 antibodies induce apoptosis of activated human T cells. J. Immunol. 165, 6205–6213 (2000).
Wesselborg, S., Janssen, O. & Kabelitz, D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J. Immunol. 150, 4338–4345 (1993).
Chatenoud, L. et al. Human in vivo antigenic modulation induced by the anti-T cell OKT3 monoclonal antibody. Eur. J. Immunol. 12, 979–982 (1982).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Rights and permissions
About this article
Cite this article
Chatenoud, L. Immune therapy for type 1 diabetes mellitus—what is unique about anti-CD3 antibodies?. Nat Rev Endocrinol 6, 149–157 (2010). https://doi.org/10.1038/nrendo.2009.275
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2009.275
This article is cited by
-
Teplizumab: type 1 diabetes mellitus preventable?
European Journal of Clinical Pharmacology (2023)
-
Contrast-enhanced ultrasound measurement of pancreatic blood flow dynamics predicts type 1 diabetes progression in preclinical models
Nature Communications (2018)
-
Oligofructose as an adjunct in treatment of diabetes in NOD mice
Scientific Reports (2016)
-
Reestablishing T Cell Tolerance by Antibody-Based Therapy in Type 1 Diabetes
Archivum Immunologiae et Therapiae Experimentalis (2015)
-
Anti-TCR therapy combined with fingolimod for reversal of diabetic hyperglycemia by β cell regeneration in the LEW.1AR1-iddm rat model of type 1 diabetes
Journal of Molecular Medicine (2014)