Key Points
-
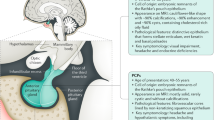
The clinical, neuroradiological and surgical definition of hypothalamic involvement is a fundamental factor related to poor postoperative outcome, progressive obesity and neuropsychological impairment in children after surgical removal of craniopharyngioma
-
The previously assumed 'gold-standard' objective of a primary radical removal of the lesion in all cases needs to be replaced with the new paradigm of a limited resection plus focused radiotherapy in patients with craniopharyngioma and hypothalamic lesions
-
Hypothalamic involvement and treatment-related hypothalamic lesions are associated with the highest risk of postoperative sequelae and impaired quality of survival
-
3D intensity-modulated proton beam radiotherapy has potential advantages over photon beam methods to focus and limit the radiation effects to optic and hypothalamic structures
-
Preclinical, in vivo mouse models can be used to investigate the pathogenesis of adamantinomatous craniopharyngioma and to test novel treatments
Abstract
Childhood-onset craniopharyngiomas are rare embryonic tumours of low-grade histological malignancy. Novel insights into the molecular pathogenesis of human adamantinomatous craniopharyngioma have started to unveil the possibility of testing novel treatments targeting pathogenic pathways. Hypothalamic involvement and/or treatment-related lesions result in impaired physical and social functionality and in severe neuroendocrine sequelae. Quality of survival in patients with craniopharyngioma with hypothalamic involvement is impaired by severe obesity, physical fatigue and non-optimal psychosocial development. Patients with craniopharyngioma involving hypothalamic structures have reduced 20-year overall survival, but overall and progression-free survival are not related to the degree of surgical resection. Irradiation is effective in the prevention of tumour progression and recurrence. For favourably localized craniopharyngiomas, the preferred treatment of choice is to attempt complete resection with preservation of visual, hypothalamic and pituitary function. For unfavourably localized tumours in close proximity to optic and/or hypothalamic structures, a radical neurosurgical strategy attempting complete resection is not recommended owing to potential severe sequelae. As expertise has been shown to have an impact on post-treatment morbidity, medical societies should establish criteria for adequate professional expertise for the treatment of craniopharyngioma. On the basis of these criteria, health authorities should organize the certification of centres of excellence that are authorized to treat and care for patients with this chronic disease.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Müller, H. L. Craniopharyngioma. Endocr. Rev. 35, 513–543 (2014).
Müller, H. L. Consequences of craniopharyngioma surgery in children. J. Clin. Endocrinol. Metab. 96, 1981–1991 (2011).
Müller, H. L. Craniopharyngioma and hypothalamic injury: latest insights into consequent eating disorders and obesity. Curr. Opin. Endocrinol. Diabetes Obes. 23, 81–89 (2016). This article reviews the broad range of neuroendocrine disorders in patients with childhood-onset craniopharyngioma.
Müller, H. L. Risk-adapted treatment and follow-up management in childhood-onset craniopharyngioma. Expert Rev. Neurother. 16, 535–548 (2016).
Bunin, G. R. et al. The descriptive epidemiology of craniopharyngioma. J. Neurosurg. 89, 547–551 (1998).
Olsson, D. S., Andersson, E., Bryngelsson, I. L., Nilsson, A. G. & Johannsson, G. Excess mortality and morbidity in patients with craniopharyngioma, especially in patients with childhood onset: a population-based study in Sweden. J. Clin. Endocrinol. Metab. 100, 467–474 (2015).
Erfurth, E. M., Holmer, H. & Fjalldal, S. B. Mortality and morbidity in adult craniopharyngioma. Pituitary 16, 46–55 (2013).
Müller, H. L. et al. Longitudinal study on growth and body mass index before and after diagnosis of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 89, 3298–3305 (2004).
Sklar, C. A. Craniopharyngioma: endocrine abnormalities at presentation. Pediatr. Neurosurg. 21 (Suppl. 1), 18–20 (1994).
Hoffmann, A. et al. History before diagnosis in childhood craniopharyngioma: associations with initial presentation and long-term prognosis. Eur. J. Endocrinol. 173, 853–862 (2015).
Steno, J., Malacek, M. & Bizik, I. Tumor–third ventricular relationships in supradiaphragmatic craniopharyngiomas: correlation of morphological, magnetic resonance imaging, and operative findings. Neurosurgery 54, 1051–1058 (2004).
Prieto, R., Pascual, J. M. & Barrios, L. Optic chiasm distortions caused by craniopharyngiomas: clinical and magnetic resonance imaging correlation and influence on visual outcome. World Neurosurg. 83, 500–529 (2015).
Pascual, J. M., Prieto, R., Carrasco, R. & Barrios, L. Displacement of mammillary bodies by craniopharyngiomas involving the third ventricle: surgical–MRI correlation and use in topographical diagnosis. J. Neurosurg. 119, 381–405 (2013).
Saeki, N. et al. Heavily T2 weighted MR images of anterior optic pathways in patients with sellar and parasellar tumours — prediction of surgical anatomy. Acta Neurochir. (Wien) 144, 25–35 (2002).
Xie, T. et al. 3D-FIESTA MR images are useful in the evaluation of the endoscopic expanded endonasal approach for midline skull-base lesions. Acta Neurochir. (Wien) 153, 12–18 (2011).
Sekine, S. et al. Craniopharyngiomas of adamantinomatous type harbor β-catenin gene mutations. Am. J. Pathol. 161, 1997–2001 (2002).
Kato, K. et al. Possible linkage between specific histological structures and aberrant reactivation of the Wnt pathway in adamantinomatous craniopharyngioma. J. Pathol. 203, 814–821 (2004).
Buslei, R. et al. Common mutations of β-catenin in adamantinomatous craniopharyngiomas but not in other tumours originating from the sellar region. Acta Neuropathol. 109, 589–597 (2005).
Brastianos, P. K. et al. Exome sequencing identifies BRAF mutations in papillary craniopharyngiomas. Nat. Genet. 46, 161–165 (2014).
Holsken, A. et al. Adamantinomatous and papillary craniopharyngiomas are characterized by distinct epigenomic as well as mutational and transcriptomic profiles. Acta Neuropathol. Commun. 4, 20 (2016).
Larkin, S. J., Preda, V., Karavitaki, N., Grossman, A. & Ansorge, O. BRAF V600E mutations are characteristic for papillary craniopharyngioma and may coexist with CTNNB1-mutated adamantinomatous craniopharyngioma. Acta Neuropathol. 127, 927–929 (2014).
Martinez-Barbera, J. P. & Buslei, R. Adamantinomatous craniopharyngioma: pathology, molecular genetics and mouse models. J. Pediatr. Endocrinol. Metab. 28, 7–17 (2015). This article reviews novel findings on molecular genetics and a mouse model for adamantinomatous craniopharyngioma.
Gaston-Massuet, C. et al. Increased Wingless (Wnt) signaling in pituitary progenitor/stem cells gives rise to pituitary tumors in mice and humans. Proc. Natl Acad. Sci. USA 108, 11482–11487 (2011).
Holsken, A. et al. Target gene activation of the Wnt signaling pathway in nuclear β-catenin accumulating cells of adamantinomatous craniopharyngiomas. Brain Pathol. 19, 357–364 (2009).
Preda, V., Larkin, S. J., Karavitaki, N., Ansorge, O. & Grossman, A. B. The Wnt signalling cascade and the adherens junction complex in craniopharyngioma tumorigenesis. Endocr. Pathol. 26, 1–8 (2015).
Hofmann, B. M. et al. Nuclear β-catenin accumulation as reliable marker for the differentiation between cystic craniopharyngiomas and rathke cleft cysts: a clinico-pathologic approach. Am. J. Surg. Pathol. 30, 1595–1603 (2006).
Gump, J. M. et al. Identification of targets for rational pharmacological therapy in childhood craniopharyngioma. Acta Neuropathol. Commun. 3, 30 (2015).
Holsken, A., Buchfelder, M., Fahlbusch, R., Blumcke, I. & Buslei, R. Tumour cell migration in adamantinomatous craniopharyngiomas is promoted by activated Wnt-signalling. Acta Neuropathol. 119, 631–639 (2010).
Holsken, A. et al. EGFR signaling regulates tumor cell migration in craniopharyngiomas. Clin. Cancer Res. 17, 4367–4377 (2011).
Stache, C. et al. Tight junction protein claudin-1 is differentially expressed in craniopharyngioma subtypes and indicates invasive tumor growth. Neuro Oncol. 16, 256–264 (2014).
Andoniadou, C. L. et al. Identification of novel pathways involved in the pathogenesis of human adamantinomatous craniopharyngioma. Acta Neuropathol. 124, 259–271 (2012).
Gomes, D. C. et al. Sonic Hedgehog pathway is upregulated in adamantinomatous craniopharyngiomas. Eur. J. Endocrinol. 172, 603–608 (2015).
Gong, J. et al. High expression levels of CXCL12 and CXCR4 predict recurrence of adamanti-nomatous craniopharyngiomas in children. Cancer Biomark. 14, 241–251 (2014).
Martinez-Barbera, J. P. 60 years of neuroendocrinology: biology of human craniopharyngioma: lessons from mouse models. J. Endocrinol. 226, T161–T172 (2015).
Andoniadou, C. L. et al. Sox2+ stem/progenitor cells in the adult mouse pituitary support organ homeostasis and have tumor-inducing potential. Cell Stem Cell 13, 433–445 (2013).
Kode, A. et al. Leukaemogenesis induced by an activating β-catenin mutation in osteoblasts. Nature 506, 240–244 (2014).
Lujambio, A. et al. Non-cell-autonomous tumor suppression by p53. Cell 153, 449–460 (2013).
Bullard, D. E. & Bigner, D. D. Heterotransplantation of human craniopharyngiomas in athymic “nude” mice. Neurosurgery 4, 308–314 (1979).
Xu, J. et al. Angiogenesis and cell proliferation in human craniopharyngioma xenografts in nude mice. J. Neurosurg. 105, 306–310 (2006).
Stache, C. et al. Insights into the infiltrative behavior of adamantinomatous craniopharyngioma in a new xenotransplant mouse model. Brain Pathol. 25, 1–10 (2015).
Apps, J. R. et al. Imaging invasion: Micro-CT imaging of adamantinomatous craniopharyngioma highlights cell type specific spatial relationships of tissue invasion. Acta Neuropathol. Commun. 4, 57 (2016).
Pettorini, B. L. et al. The role of inflammation in the genesis of the cystic component of craniopharyngiomas. Childs Nerv. Syst. 26, 1779–1784 (2010).
Stevens, A., Kloter, I. & Roggendorf, W. Inflammatory infiltrates and natural killer cell presence in human brain tumors. Cancer 61, 738–743 (1988).
Hoffman, H. J. et al. Aggressive surgical management of craniopharyngiomas in children. J. Neurosurg. 76, 47–52 (1992).
Choux, M. & Lena, G. Bases of surgical management of craniopharyngioma in children [proceedings]. Acta Neurochir. Suppl. (Wien) 28, 348 (1979).
Pierre-Kahn, A. et al. Treatment of craniopharyngiomas in children. Retrospective analysis of 50 cases. Arch. Fr. Pediatr. 45, 163–167 (in French) (1988).
Yasargil, M. G. et al. Total removal of craniopharyngiomas. Approaches and long-term results in 144 patients. J. Neurosurg. 73, 3–11 (1990).
Caldarelli, M., di Rocco, C., Papacci, F. & Colosimo, C. Jr. Management of recurrent craniopharyngioma. Acta Neurochir. (Wien) 140, 447–454 (1998).
Zuccaro, G. Radical resection of craniopharyngioma. Childs Nerv. Syst. 21, 679–690 (2005).
DeVile, C. J., Grant, D. B., Hayward, R. D. & Stanhope, R. Growth and endocrine sequelae of craniopharyngioma. Arch. Dis. Child. 75, 108–114 (1996).
Müller, H. L. et al. Functional capacity and body mass index in patients with sellar masses — cross-sectional study on 403 patients diagnosed during childhood and adolescence. Childs Nerv. Syst. 21, 539–545 (2005).
Müller, H. L. et al. Longitudinal study on quality of life in 102 survivors of childhood craniopharyngioma. Childs Nerv. Syst. 21, 975–980 (2005).
Puget, S. et al. Pediatric craniopharyngiomas: classification and treatment according to the degree of hypothalamic involvement. J. Neurosurg. 106, 3–12 (2007). This article reports on a currently used grading system for hypothalamic involvement in childhood-onset craniopharyngioma.
Sainte-Rose, C. et al. Craniopharyngioma: the pendulum of surgical management. Childs Nerv. Syst. 21, 691–695 (2005).
Mallucci, C. et al. Management of craniopharyngioma: the Liverpool experience following the introduction of the CCLG guidelines. Introducing a new risk assessment grading system. Childs Nerv. Syst. 28, 1181–1192 (2012).
Bartels, U., Laperriere, N., Bouffet, E. & Drake, J. Intracystic therapies for cystic craniopharyngioma in childhood. Front. Endocrinol. (Lausanne) 3, 39 (2012). This is a comprehensive review on intracystic treatment options in childhood-onset craniopharyngioma.
Dastoli, P. A. et al. Cystic craniopharyngioma: intratumoral chemotherapy with α interferon. Arq. Neuropsiquiatr. 69, 50–55 (2011).
Tatreau, J. R. et al. Anatomical considerations for endoscopic endonasal skull base surgery in pediatric patients. Laryngoscope 120, 1730–1737 (2010).
Ali, Z. S. et al. Suprasellar pediatric craniopharyngioma resection via endonasal endoscopic approach. Childs Nerv. Syst. 29, 2065–2070 (2013).
Campbell, P. G. et al. Endocrinological and ophthalmological consequences of an initial endonasal endoscopic approach for resection of craniopharyngiomas. Neurosurg. Focus 28, E8 (2010).
Locatelli, D. et al. Endoscopic endonasal transsphenoidal surgery for sellar tumors in children. Int. J. Pediatr. Otorhinolaryngol. 74, 1298–1302 (2010).
Fahlbusch, R., Honegger, J., Paulus, W., Huk, W. & Buchfelder, M. Surgical treatment of craniopharyngiomas: experience with 168 patients. J. Neurosurg. 90, 237–250 (1999).
Zona, G. & Spaziante, R. Management of cystic craniopharyngiomas in childhood by a transsphenoidal approach. J. Pediatr. Endocrinol. Metab. 19 (Suppl. 1), 381–388 (2006).
Elliott, R. E., Jane, J. A. Jr & Wisoff, J. H. Surgical management of craniopharyngiomas in children: meta-analysis and comparison of transcranial and transsphenoidal approaches. Neurosurgery 69, 630–643 (2011).
Prieto, R. et al. Predictive factors for craniopharyngioma recurrence: a systematic review and illustrative case report of a rapid recurrence. World Neurosurg. 79, 733–749 (2013).
Steno, J. Microsurgical topography of craniopharyngiomas. Acta Neurochir. Suppl. (Wien) 35, 94–100 (1985).
Pascual, J. M., Prieto, R. & Carrasco, R. Infundibulo-tuberal or not strictly intraventricular craniopharyngioma: evidence for a major topographical category. Acta Neurochir. (Wien) 153, 2403–2425 (2011).
Pan, J. et al. Intraventricular craniopharyngioma: morphological analysis and outcome evaluation of 17 cases. Acta Neurochir. (Wien) 153, 773–784 (2011).
Qi, S. et al. Anatomic relations of the arachnoidea around the pituitary stalk: relevance for surgical removal of craniopharyngiomas. Acta Neurochir. (Wien) 153, 785–796 (2011).
Tomita, T. & Bowman, R. M. Craniopharyngiomas in children: surgical experience at Children's Memorial Hospital. Childs Nerv. Syst. 21, 729–746 (2005).
Wang, K. C., Hong, S. H., Kim, S. K. & Cho, B. K. Origin of craniopharyngiomas: implication on the growth pattern. Childs Nerv. Syst. 21, 628–634 (2005).
Kiehna, E. N. & Merchant, T. E. Radiation therapy for pediatric craniopharyngioma. Neurosurg. Focus 28, E10 (2010). This is a comprehensive review of radio-oncological treatment techniques and strategies in childhood-onset craniopharyngioma.
Dunbar, S. F. et al. Stereotactic radiotherapy for pediatric and adult brain tumors: preliminary report. Int. J. Radiat. Oncol. Biol. Phys. 30, 531–539 (1994).
Merchant, T. E. et al. Disease control after reduced volume conformal and intensity modulated radiation therapy for childhood craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 85, e187–e192 (2013).
Di Pinto, M., Conklin, H. M., Li, C. & Merchant, T. E. Learning and memory following conformal radiation therapy for pediatric craniopharyngioma and low-grade glioma. Int. J. Radiat. Oncol. Biol. Phys. 84, e363–e369 (2012).
Beltran, C., Roca, M. & Merchant, T. E. On the benefits and risks of proton therapy in pediatric craniopharyngioma. Int. J. Radiat. Oncol. Biol. Phys. 82, e281–e287 (2012).
Merchant, T. E. et al. Necrosis, vasculopathy and neurological complications after proton therapy for childhood craniopharyngioma: Results from a prospective trial and a photon cohort comparison [abstract 269]. Int. J. Radiat. Oncol. Biol. Phys. 96 (Suppl.), S120–S121 (2016).
Bishop, A. J. et al. Proton beam therapy versus conformal photon radiation therapy for childhood craniopharyngioma: multi-institutional analysis of outcomes, cyst dynamics, and toxicity. Int. J. Radiat. Oncol. Biol. Phys. 90, 354–361 (2014).
Uh, J. et al. Effects of surgery and proton therapy on cerebral white matter of craniopharyngioma patients. Int. J. Radiat. Oncol. Biol. Phys. 93, 64–71 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01419067?term=NCT01419067&rank=1 (2016).
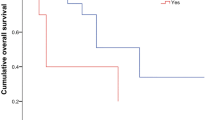
Sterkenburg, A. S. et al. Survival, hypothalamic obesity, and neuropsychological/psychosocial status after childhood-onset craniopharyngioma: newly reported long-term outcomes. Neuro Oncol. 17, 1029–1038 (2015). This article describes a20-years follow-up analysis of 485 patients with childhood-onset craniopharyngioma, which shows impaired overall survival in patients with hypothalamic involvement and similar relapse and progression rates with regard to different degrees of resection (gross total resection versus incomplete resection).
Daubenbuchel, A. M. et al. Hydrocephalus and hypothalamic involvement in pediatric patients with craniopharyngioma or cysts of Rathke's pouch: impact on long-term prognosis. Eur. J. Endocrinol. 172, 561–569 (2015).
Pickering, L. et al. Sleep-wake and melatonin pattern in craniopharyngioma patients. Eur. J. Endocrinol. 170, 873–884 (2014).
Müller, H. L. Increased daytime sleepiness in patients with childhood craniopharyngioma and hypothalamic tumor involvement: review of the literature and perspectives. Int. J. Endocrinol. 2010, 519607 (2010).
Müller, H. L. et al. Melatonin treatment in obese patients with childhood craniopharyngioma and increased daytime sleepiness. Cancer Causes Control 17, 583–589 (2006).
Romijn, J. A. Pituitary diseases and sleep disorders. Curr. Opin. Endocrinol. Diabetes Obes. 23, 345–351 (2016).
Hoffmann, A., Postma, F. P., Sterkenburg, A. S., Gebhardt, U. & Müller, H. L. Eating behavior, weight problems and eating disorders in 101 long-term survivors of childhood-onset craniopharyngioma. J. Pediatr. Endocrinol. Metab. 28, 35–43 (2015).
Heymsfield, S. B. et al. Hyperphagia: current concepts and future directions proceedings of the 2nd international conference on hyperphagia. Obesity (Silver Spring) 22, S1–S17 (2014).
Roth, C. L. et al. Functional neuroimaging in craniopharyngioma: a useful tool to better understand hypothalamic obesity? Obes. Facts 5, 243–253 (2012).
Ozyurt, J. et al. Neuropsychological outcome in patients with childhood craniopharyngioma and hypothalamic involvement. J. Pediatr. 164, 876–881.e4 (2014).
Ozyurt, J. et al. Remote effects of hypothalamic lesions in the prefrontal cortex of craniopharygioma patients. Neurobiol. Learn. Mem. 111, 71–80 (2014).
Honegger, J., Buchfelder, M. & Fahlbusch, R. Surgical treatment of craniopharyngiomas: endocrinological results. J. Neurosurg. 90, 251–257 (1999).
Hoffmann, A. et al. Nonalcoholic fatty liver disease and fatigue in long-term survivors of childhood-onset craniopharyngioma. Eur. J. Endocrinol. 173, 389–397 (2015). This is the first report on the incidence of and risk factors for nonalcoholic fatty liver diseasein patients with childhood-onset craniopharyngioma.
Roemmler-Zehrer, J. et al. Specific behaviour, mood and personality traits may contribute to obesity in patients with craniopharyngioma. Clin. Endocrinol. (Oxf.) 82, 106–114 (2015).
Zada, G., Kintz, N., Pulido, M. & Amezcua, L. Prevalence of neurobehavioral, social, and emotional dysfunction in patients treated for childhood craniopharyngioma: a systematic literature review. PLoS ONE 8, e76562 (2013).
Fjalldal, S. et al. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J. Clin. Endocrinol. Metab. 98, 3253–3262 (2013). This is a study on the association between hypothalamic involvement and neuropsychological outcome in childhood craniopharyngioma.
Crespo, I., Santos, A. & Webb, S. M. Quality of life in patients with hypopituitarism. Curr. Opin. Endocrinol. Diabetes Obes. 22, 306–312 (2015).
Crespo, I., Valassi, E., Santos, A. & Webb, S. M. Health-related quality of life in pituitary diseases. Endocrinol. Metab. Clin. North. Am. 44, 161–170 (2015).
Ozyurt, J., Müller, H. L. & Thiel, C. M. A systematic review of cognitive performance in patients with childhood craniopharyngioma. J. Neurooncol. 125, 9–21 (2015). This review reportsneuropsychological outcomes in long-term survivors of childhood-onset craniopharyngioma, with special regard to hypothalamic involvement.
Pereira, A. M. et al. High prevalence of long-term cardiovascular, neurological and psychosocial morbidity after treatment for craniopharyngioma. Clin. Endocrinol. (Oxf.) 62, 197–204 (2005).
Haliloglu, B. & Bereket, A. Hypothalamic obesity in children: pathophysiology to clinical management. J. Pediatr. Endocrinol. Metab. 28, 503–513 (2015).
Müller, H. L. Paediatrics: surgical strategy and quality of life in craniopharyngioma. Nat. Rev. Endocrinol. 9, 447–449 (2013).
Müller, H. L. Risk-adapted, long-term management in childhood-onset craniopharyngioma. Pituitary http://dx.doi.org/10.1007/s11102-016-0751-0 (2016).
Elfers, C. T. & Roth, C. L. Effects of methylphenidate on weight gain and food intake in hypothalamic obesity. Front. Endocrinol. (Lausanne) 2, 78 (2011).
Zoicas, F., Droste, M., Mayr, B., Buchfelder, M. & Schofl, C. GLP-1 analogues as a new treatment option for hypothalamic obesity in adults: report of nine cases. Eur. J. Endocrinol. 168, 699–706 (2013).
Daubenbuchel, A. M. et al. Oxytocin in survivors of childhood-onset craniopharyngioma. Endocrine 54, 524–531 (2016). This is the first report on oxytocin saliva concentrations in childhood-onset craniopharyngioma with regard to different grades of hypothalamic involvement.
Bretault, M. et al. Clinical review: bariatric surgery following treatment for craniopharyngioma: a systematic review and individual-level data meta-analysis. J. Clin. Endocrinol. Metab. 98, 2239–2246 (2013).
Gatta, B. et al. Is bariatric surgery really inefficient in hypothalamic obesity? Clin. Endocrinol. (Oxf.) 78, 636–638 (2013).
Müller, H. L., Gebhardt, U., Maroske, J. & Hanisch, E. Long-term follow-up of morbidly obese patients with childhood craniopharyngioma after laparoscopic adjustable gastric banding (LAGB). Klin. Padiatr. 223, 372–373 (2011).
de Vile, C. J. et al. Obesity in childhood craniopharyngioma: relation to post-operative hypothalamic damage shown by magnetic resonance imaging. J. Clin. Endocrinol. Metab. 81, 2734–2737 (1996).
Elowe-Gruau, E. et al. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J. Clin. Endocrinol. Metab. 98, 2376–2382 (2013). This is the first report on the effects of hypothalamus-sparing surgical strategies on outcome in childhood craniopharyngioma.
Müller, H. L. et al. Xanthogranuloma, Rathke's cyst, and childhood craniopharyngioma: results of prospective multinational studies of children and adolescents with rare sellar malformations. J. Clin. Endocrinol. Metab. 97, 3935–3943 (2012).
Müller, H. L. et al. Post-operative hypothalamic lesions and obesity in childhood craniopharyngioma: results of the multinational prospective trial KRANIOPHARYNGEOM 2000 after 3-year follow-up. Eur. J. Endocrinol. 165, 17–24 (2011). This prospective study shows that hypothalamic lesions are associated with poor outcome and obesity in childhood-onset craniopharyngioma.
Van Gompel, J. J., Nippoldt, T. B., Higgins, D. M. & Meyer, F. B. Magnetic resonance imaging-graded hypothalamic compression in surgically treated adult craniopharyngiomas determining postoperative obesity. Neurosurg. Focus 28, E3 (2010).
Elliott, R. E., Sands, S. A., Strom, R. G. & Wisoff, J. H. Craniopharyngioma Clinical Status Scale: a standardized metric of preoperative function and posttreatment outcome. Neurosurg. Focus 28, E2 (2010).
Steno, J., Bizik, I., Steno, A. & Matejcik, V. Craniopharyngiomas in children: how radical should the surgeon be? Childs Nerv. Syst. 27, 41–54 (2011).
Roth, C. L. et al. Semiquantitative analysis of hypothalamic damage on MRI predicts risk for hypothalamic obesity. Obesity (Silver Spring) 23, 1226–1233 (2015).
Mortini, P. et al. Magnetic resonance imaging as predictor of functional outcome in craniopharyngiomas. Endocrine 51, 148–162 (2016). This is a study on MRI and clinical findings in 47 patients with childhood-onset craniopharyngioma, aiming atidentifying objective radiological criteria as preoperative prognostic factors of hypothalamic damage and including a comparison of the results with regard to current grading systems for hypothalamic involvement and/or damage.
Garre, M. L. & Cama, A. Craniopharyngioma: modern concepts in pathogenesis and treatment. Curr. Opin. Pediatr. 19, 471–479 (2007).
Müller, H. L. Childhood craniopharyngioma — current concepts in diagnosis, therapy and follow-up. Nat. Rev. Endocrinol. 6, 609–618 (2010).
De Vile, C. J. et al. Management of childhood craniopharyngioma: can the morbidity of radical surgery be predicted? J. Neurosurg. 85, 73–81 (1996).
Müller, H. L. Craniopharyngioma: long-term consequences of a chronic disease. Expert Rev. Neurother. 15, 1241–1244 (2015).
Sanford, R. A. Craniopharyngioma: results of survey of the American Society of Pediatric Neurosurgery. Pediatr. Neurosurg. 21 (Suppl. 1), 39–43 (1994).
Boop, F. A. Craniopharyngioma. J. Neurosurg. 106, 1–2 (2007).
Hankinson, T. C. et al. Patterns of care for craniopharyngioma: survey of members of the American Association of Neurological Surgeons. Pediatr. Neurosurg. 49, 131–136 (2013).
Hoffmann, A. et al. Childhood craniopharyngioma — changes of treatment strategies in the trials KRANIOPHARYNGEOM 2000/2007. Klin. Padiatr. 226, 161–168 (2014).
Rath, S. R. et al. Childhood craniopharyngioma: 20-year institutional experience in Western Australia. J. Paediatr. Child Health 49, 403–408 (2013).
Lo, A. C. et al. Long-term outcomes and complications in patients with craniopharyngioma: the British Columbia Cancer Agency experience. Int. J. Radiat. Oncol. Biol. Phys. 88, 1011–1018 (2014).
Zaidi, H. A., Chapple, K. & Little, A. S. National treatment trends, complications, and predictors of in-hospital charges for the surgical management of craniopharyngiomas in adults from 2007 to 2011. Neurosurg. Focus 37, E6 (2014).
Müller, H. L. Childhood craniopharyngioma: treatment strategies and outcomes. Expert Rev. Neurother. 14, 187–197 (2014).
Flitsch, J., Aberle, J. & Burkhardt, T. Surgery for pediatric craniopharyngiomas: is less more? J. Pediatr. Endocrinol. Metab. 28, 27–33 (2015).
Buchfelder, M., Schlaffer, S. M., Lin, F. & Kleindienst, A. Surgery for craniopharyngioma. Pituitary 16, 18–25 (2013).
Mortini, P., Gagliardi, F., Boari, N. & Losa, M. Surgical strategies and modern therapeutic options in the treatment of craniopharyngiomas. Crit. Rev. Oncol. Hematol. 88, 514–529 (2013).
Ali, Z. S. et al. Comparative effectiveness of treatment options for pediatric craniopharyngiomas. J. Neurosurg. Pediatr. 13, 178–188 (2014).
Ogawa, Y., Kawaguchi, T. & Tominaga, T. Outcome and mid-term prognosis after maximum and radical removal of craniopharyngiomas with the priority to the extended transsphenoidal approach — a single center experience. Clin. Neurol. Neurosurg. 125, 41–46 (2014).
Raza, S. M. & Schwartz, T. H. How to achieve the best possible outcomes in the management of retroinfundibular craniopharyngiomas? World Neurosurg. 82, 614–616 (2014).
Daubenbuchel, A. M. & Müller, H. L. Neuroendocrine disorders in pediatric craniopharyngioma patients. J. Clin. Med. 4, 389–413 (2015).
Flitsch, J., Müller, H. L. & Burkhardt, T. Surgical strategies in childhood craniopharyngioma. Front. Endocrinol. (Lausanne) 2, 96 (2011).
Acknowledgements
H.L.M. acknowledges support from the German Childhood Cancer Foundation, Bonn, Germany.
Author information
Authors and Affiliations
Contributions
H.L.M., T.E.M., S.P. and J.-P.M.-B. researched data for the article, made substantial contributions to discussions of the content, wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Müller, H., Merchant, T., Puget, S. et al. New outlook on the diagnosis, treatment and follow-up of childhood-onset craniopharyngioma. Nat Rev Endocrinol 13, 299–312 (2017). https://doi.org/10.1038/nrendo.2016.217
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.217
This article is cited by
-
Peripheral oxytocin levels are linked to hypothalamic gray matter volume in autistic adults: a cross-sectional secondary data analysis
Scientific Reports (2024)
-
Visual outcomes after treatment of craniopharyngioma in children: A systematic review
Child's Nervous System (2024)
-
Diagnostic support in pediatric craniopharyngioma using deep learning
Child's Nervous System (2024)
-
Molecular biological features of cyst wall of adamantinomatous craniopharyngioma
Scientific Reports (2023)
-
Kinderwunschbehandlung nach Kraniopharyngeom in der Kindheit
Gynäkologische Endokrinologie (2023)