Key Points
-
Asian Indian individuals have a high predisposition to type 2 diabetes mellitus (T2DM), which develops at a younger age and lower BMI than in western countries
-
Asian Indian people with T2DM tend to have a higher risk of coronary artery disease and, possibly, a lower risk of microvascular complications compared with white individuals
-
Since the 1960s, the prevalence of diabetes mellitus in India has increased in both the urban and rural areas of the country and has spread to involve individuals from all socioeconomic strata
-
Management of diabetes mellitus in India faces many challenges but novel interventions using readily available resources and technology promise to revolutionise care
-
Understanding how India deals with diabetes mellitus can help other low-income and middle-income countries in the treatment of this devastating disease
Abstract
India is one of the epicentres of the global diabetes mellitus pandemic. Rapid socioeconomic development and demographic changes, along with increased susceptibility for Indian individuals, have led to the explosive increase in the prevalence of diabetes mellitus in India over the past four decades. Type 2 diabetes mellitus in Asian Indian people is characterized by a young age of onset and occurrence at low levels of BMI. Available data also suggest that the susceptibility of Asian Indian people to the complications of diabetes mellitus differs from that of white populations. Management of this disease in India faces multiple challenges, such as low levels of awareness, paucity of trained medical and paramedical staff and unaffordability of medications and services. Novel interventions using readily available resources and technology promise to revolutionise the care of patients with diabetes mellitus in India. As many of these challenges are common to most developing countries of the world, the lessons learnt from India's experience with diabetes mellitus are likely to be of immense global relevance. In this Review, we discuss the epidemiology of diabetes mellitus and its complications in India and outline the advances made in the country to ensure adequate care. We make specific references to novel, cost-effective interventions, which might be of relevance to other low-income and middle-income countries of the world.
Similar content being viewed by others
Main
Diabetes mellitus, a chronic metabolic noncommunicable disease (NCD), has attained epidemic proportions worldwide. As of 2015, >415 million adults have diabetes mellitus, and this number is estimated to increase to 642 million by 2040 (Ref. 1). More than 95% of all adults with diabetes mellitus have type 2 diabetes mellitus (T2DM). India is one of the epicentres of the global diabetes mellitus epidemic and has the second highest number of people with the disease in the world (∼69 million individuals as of 2015)1. Other countries of the south Asian region, such as Bangladesh, Pakistan, Sri Lanka and Nepal, also have large numbers of individuals with diabetes mellitus1. In addition, countries such as the UK, the USA, Mauritius, Fiji, Malaysia, Singapore, South Africa and countries in the Gulf region of the Middle East are home to a large diaspora of Asian Indian individuals, who have been found to have a much higher prevalence of diabetes mellitus than the native populations of the respective countries2,3,4.
Asian Indian people, which we broadly define as individuals originating from the Indian subcontinent (the countries of India, Pakistan, Bangladesh, Sri Lanka, Afghanistan, Nepal, Bhutan and the Maldives)5 constitute >17% of the world's population and also have a specific phenotype, characterized by high levels of intra-abdominal fat and insulin resistance in spite of a low BMI, which predisposes them to T2DM and premature coronary heart disease6. Learning about the patterns and behaviour of diabetes mellitus in this ethnic group is of the utmost importance. Fortunately, in parallel with the increase in the prevalence of diabetes mellitus in India, research activities on this disease and its complications have increased. In this Review, we discuss the epidemiology and unique features of diabetes mellitus and its complications in India. We will also highlight the major challenges in the delivery of optimal care for patients with diabetes mellitus and suggest possible solutions.
Epidemiology
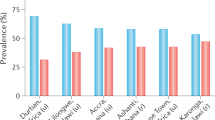
The first formal studies to evaluate the prevalence of diabetes mellitus in India did not occur until the middle of the twentieth century. By the end of the 1960s, seven studies had been published detailing the prevalence of the disease7,8,9,10,11,12. Subsequently, numerous single-centre and multicentre studies on the epidemiology of diabetes mellitus in various parts of the country have been published. Notwithstanding the caveat that these studies have used varying methodologies, sampling techniques and diagnostic criteria, their results suggest a clear increasing trend in the prevalence of diabetes mellitus. For example, this trend has been most markedly visible in the southern Indian city of Chennai, where the results of a series of studies conducted from 1989 to 2004 showed a 72% increase in the prevalence of the disease13 (Fig. 1; Supplementary information 1 (Figure)).
a | The prevalence of diabetes mellitus in rural and urban populations in India between 1966 and 1975. b | The prevalence of diabetes mellitus in rural and urban populations in India in 2014. These figures do not differentiate between different types of diabetes mellitus; however, according to the International Diabetes Foundation Atlas ∼95% of adult individuals with the disease have type 2 diabetes mellitus. An expanded version of this figure is included in Supplementary information S1 (figure).
The first multicentre study on diabetes mellitus in India was initiated by the Indian Council of Medical Research (ICMR) in 1971. This study estimated the prevalence of diabetes mellitus in six cities and surrounding villages in India (Ahmedabad, Kolkata, Cuttack, Delhi, Pune and Trivandrum). The prevalence of the disease were found to be 2.1% in the urban areas and 1.5% in the rural areas14. More than two decades later, the National Urban Diabetes Study sampled individuals from six major metropolitan cities of India and reported prevalences ranging from 9.3% in Mumbai to 16.6% in Hyderabad15 (Fig. 1). At around the same time, the Prevalence of Diabetes in India study evaluated the prevalence of diabetes mellitus in small towns and villages of India, which was found to be 5.9% and 2.7%, respectively16.
Until 2011, prevalence estimates for diabetes mellitus in India from the International Diabetes Federation (IDF) were based on the results of these, and other smaller, studies17. However, none of these studies could be considered fully representative of India as a whole. For instance, the National Urban Diabetes Study omitted the rural areas completely and the Prevalence of Diabetes in India Study did not study the large metropolitan cities.
The ongoing ICMR–India Diabetes (ICMR–INDIAB) study aims to address this knowledge gap by estimating the prevalence of diabetes mellitus in India, using uniform sampling techniques and diagnostic criteria in a representative sample of individuals from rural and urban areas of all 29 states of India18. From the results of phase I of the study (covering four regions of the country: Tamil Nadu, Maharashtra, Jharkhand and Chandigarh), it was estimated that 62 million individuals had diabetes mellitus and 77 million had prediabetes (that is, impaired glucose tolerance and impaired fasting glucose according to the WHO criteria) in India in 2011 (Ref. 19). These results led the IDF to revise their estimates of the number of people with the disease in India from 50 million (in the 2009 edition of the IDF Atlas) to 61.9 million (in the 2011 edition)20. These numbers are, of course, not static; and the 2015 update of the Atlas estimates that 69.2 million people in India will have diabetes mellitus1. Table 1 outlines some of the single-centre studies on the prevalence of diabetes mellitus in India, and Table 2 (Refs 14,15,16,17,18,19) shows the multicentre studies in which a definite increase in prevalence of diabetes mellitus was observed over time.
What are the most likely reasons for such a huge increase in the prevalence of diabetes mellitus in India? Have diagnostic criteria changed; is sampling and detection better; is the increase in population with improved longevity and demographic changes a factor; have risk factors for the disease increased; or are lifestyle changes leading to obesity, unhealthy diet and physical inactivity? In all likelihood, all of these factors probably contribute to the prevalence of the disease, and identifying the individual causes is difficult. More detailed studies need to be done to understand the impact of each of these factors on the rising diabetes mellitus epidemic in India.
Are Asian Indian people more prone to T2DM?
Migrant Asian Indian individuals in various parts of the world such as Mauritius, Fiji and the UK2,3,4, have a higher prevalence of T2DM than the native populations of those countries3,4,21,22,23,24,25,26,27,28 (Table 3). In addition, studies conducted in the past few years have reported that the incidence of T2DM in Asian Indian people is among the highest in the world, exceeded only by some isolated and homogenous populations such as the Pima Indian people and the Pacific Islanders of Nauru29.
This increased propensity to develop T2DM occurs despite Asian Indian individuals being younger, and in many cases leaner, than non-Indian counterparts when diagnosed with the disease in these studies30. In a study of 24,335 individuals from 11 countries, Asian Indian people were found to have the highest prevalence of diabetes mellitus, and the peak prevalence of the disease was reached ∼10 years earlier in Asian Indian individuals compared with Chinese people and Japanese people31. According to the ICMR–INDIAB study, the 'take-off point' for increased prevalence of T2DM among Asian Indian individuals is 25–34 years, clearly a decade or two earlier than in western populations19. These findings have led to the concept of a specific 'Asian Indian phenotype', a collection of clinical and biochemical features that dispose Asian Indian people to a higher risk of T2DM than individuals from other major ethnic groups (Fig. 2)6. For example, for a given BMI, Asian Indian individuals have higher waist circumference, higher waist-hip ratios, more subcutaneous and visceral fat and more insulin resistance than individuals of European origin32. Asian Indian people also have a high prevalence of the metabolic syndrome (20–32%)33 with some specific findings as outlined here34. Asian Indian people have been consistently shown to have higher fasting insulin levels than white individuals35,36. Indeed, hyperinsulinaemia has even been detected in the cord blood of otherwise healthy Asian Indian infants, even though they were on average lighter than their white counterparts37. Exposure to high levels of insulin resistance from early in life is thought to render the pancreatic β cell less capable of compensating for the decline in insulin sensitivity that normally occurs with age, which might contribute to the early development of hyperglycaemia and T2DM38. In a 2013 study from southern India, β-cell dysfunction (as measured by the insulin disposition index) was found to be prominent in Asian Indian patients with even mild dysglycaemia (that is, impaired fasting glucose, impaired glucose tolerance or both)39.
The Asian Indian phenotype can lead to increased prevalence of T2DM, which has a unique clinical profile. Patients with T2DM in India also have unique management challenges.
What could be the reason for the development of this unhealthy phenotype in a substantial proportion of one of the world's largest ethnic groups? Investigators in several studies have tried to assess the role of population-specific genetic and epigenetic factors in the predisposition of Asian Indian people to T2DM. However, the paucity of large-scale genome-wide association studies (GWAS) in the Asian Indian population prevents any firm conclusions. Of the GWAS that have been conducted in Asian Indian populations many, but not all, of the susceptibility loci for T2DM identified in European populations have been found. For example, a GWAS in an Asian Indian population (the results of which were published in 2011) identified six novel T2DM susceptibility loci (in GRB14, ST6GAL1, VPS26A, HMG20A, AP3S2 and HNF4A)40. In a population of 2,465 individuals belonging to two ethnic groups in India comprising 7,221 Indo-Europeans and 2,849 Dravidians from New Delhi, Jaipur and Chennai, the TMEM163 gene was shown to have a genome-wide significant association with T2DM41. In a GWAS of Punjabi Sikh individuals from India, a single nucleotide polymorphism within the SGCG gene had significant genome-wide association with T2DM42. Notably, certain genotypes (such as the Pro12Ala polymorphism of the PPARγ gene) that protect against development of T2DM in white individuals, do not seem to do so among Asian Indian patients43. Considering the vast ethnic diversity of India, more GWAS need to be performed from other parts of the country to elucidate the genetic basis of T2DM in this population.
Although genetic factors undoubtedly predispose Asian Indian people to the development of T2DM, the explosion in the prevalence of this disease in India over the past four decades cannot be fully explained by these factors alone — major changes in the genetic makeup of a population cannot occur within such a short time span. Environmental factors seem to have a far more important role in the development and propagation of the T2DM epidemic in India. In this context, the role of early life (or antenatal) factors is important. Maternal malnutrition (and its attendant consequence of small-for-gestational age babies) has been suggested as a major driver of the diabetes mellitus epidemic in India. Infants born to undernourished women have higher levels of adiposity in spite of their small size than infants born to adequately nourished mothers44. Low maternal levels of vitamin B12, particularly when coupled with normal to high folate levels, are associated with adiposity and insulin resistance in the offspring45. Maternal undernutrition has also been associated with diminished β-cell mass in experimental animals, but to what extent this finding applies to humans is unclear46. Small-for-gestational age babies who experience rapid weight gain (known as adiposity rebound) after the age of 2 years have a particularly high risk of developing impaired glucose tolerance during adolescence47. The accelerated increase in BMI in children in India has been suggested to be a fairly new phenomenon, driven by the process of nutrition transition, and this upward trajectory of BMI starting in early childhood might explain, at least in part, the current epidemic of T2DM in India.
In the past 40 years, and particularly since economic liberalization in 1991, the lifestyle of the average Asian Indian person has undergone far-reaching changes. Vehicle ownership has more than quadrupled, mobile telephones and computers have become widespread and occupations have become more sedentary and less labour-intensive than in previous years48. These developments have led to a drastic decline in occupational physical activity levels of people in India, which has not been compensated for by an increase in the levels of recreational physical activity. In fact, data show that <10% of the Indian population performs any recreational physical activity at all49. Improvement in food security and increased disposable income have shifted the consumption patterns of Indian individuals away from coarse grains and millets to highly refined white rice and wheat flour as a staple50. In a study in the urban population of Chennai, India, individuals in the highest quartile of refined cereal intake were found to have 7.83-fold higher odds of developing the metabolic syndrome and a 7.9% increased prevalence of elevated fasting levels of glucose compared with individuals in the lowest quartile51. In addition, increased consumption of junk food and beverages sweetened with sugar has the potential to further accelerate the development of diabetes mellitus.
Although these changes are still not as profound in the rural areas of India as in the cities, individuals living in rural communities are fast catching up with those in urban areas. Another important factor is large-scale migration of rural dwellers into cities in search of better opportunities. For example, in some studies, these rural-to-urban migrants have been shown to have a much higher prevalence of diabetes mellitus than their kin who stayed behind in the villages (14.3 versus 6.2%, respectively)52.
Considering these developments, the finding that the prevalence of obesity and overweight are disturbingly high in many parts of India is unsurprising53,54, even among schoolchildren55. As noted earlier in the text, being overweight in childhood and adolescence probably adds to the risk of insulin resistance conferred by low birth weight and sets the stage for development of T2DM by early adulthood.
In fact, Asian Indian people living in their native country now, for the first time, have a higher prevalence of T2DM than their counterparts who have moved abroad (for example, to the USA), with an age-adjusted prevalence among adults aged 40–84 years of 38% compared with 24% in those who have emigrated56. This finding indicates that the epidemic of T2DM is now firmly established in India and that the numbers can be expected to further increase in the near future. Even more worryingly, within India, T2DM seems to be moving into the lower income groups, who cannot afford to pay for monitoring and treatment57,58.
Other forms of diabetes mellitus
Type 1 diabetes mellitus
Type 1 diabetes mellitus (T1DM) is uncommon in India compared with western nations. Nevertheless, the incidence is higher than in many other Asian countries, including China; estimates indicate that every fifth child in the world with T1DM is Indian, compared with every seventh adult with T2DM1,59.
The IDF estimates that three new cases of T1DM per 100,000 children aged 0–14 years occur in India every year1. Wide regional variation seems to exist, with studies from Karnataka, Chennai and Karnal (a district in the state of Haryana) reporting incidence rates of 17.93, 3.2 and 10.2 cases per 100,000 children, respectively60,61,62. However, these numbers are higher than the estimated incidence in China (0.1 per 100,000), but still low compared with European regions such as Sardinia and Finland (36.8 and 36.5 per 100,000, respectively)63.
T1DM in India tends to be infrequently associated with positive titres of pancreatic autoantibodies and is therefore classified as idiopathic (T1b) diabetes mellitus64. The human leukocyte antigen (HLA) associations with T1DM are also different in Asian Indian individuals compared with those described for white populations. For example, in a study of North Indian children with T1DM, the haplotypes A26-B8-DRB1*03 and Ax-B50-DRB1*03 were encountered most frequently (found in 25% each of patients). The classic haplotype A1-B8-DRB1*03 that favours autoimmunity in white individuals was found only in a minority (7.2%) of this Asian Indian population65.
Monogenic diabetes mellitus
Few data exist on the prevalence of monogenic forms of diabetes mellitus in India. In a series of 96 unrelated patients diagnosed with monogenic diabetes of the young (MODY) according to the clinical criteria of Tattersall and Fajans66, 9% were found to have mutations in HNF1A (previously known as MODY3) and 3.4% in HNF4A (previously known as MODY1)67,68. In another study from southern India, 11 mutations were found in 56 patients with clinically diagnosed MODY, of which seven were novel mutations69. Efforts to elucidate the clinical picture of monogenic diabetes mellitus in India are limited by the paucity of specialized genetic testing facilities and the considerable expenses involved.
Fibrocalculous pancreatic diabetes mellitus
Fibrocalculous pancreatic diabetes mellitus (FCPD) is an uncommon form of diabetes mellitus secondary to chronic calcific nonalcoholic pancreatitis70. FCPD used to be encountered in the southern parts of India (particularly in the states of Kerala and Tamil Nadu). However, the prevalence seems to be declining even in these states; in a series from Chennai, patients with FCPD constituted 1.6% of all patients with diabetes mellitus between 1991 and 1995, but only 0.2% between 2006 and 2010 (Refs 71,72). Patients with FCPD have recurrent episodes of abdominal pain and oily stools and an abdominal radiograph reveals pancreatic calculi70. These patients are as prone to developing microvascular complications as individuals with T2DM; however, macrovascular disease seems to be uncommon. In a study from southern India that compared the prevalences of diabetes mellitus complications in individuals with FCPD and those with T2DM, prevalences were similar for nephropathy (10.1% versus 15.0%), retinopathy (30.1% versus 37.2%) and neuropathy (20.9% versus 25.3%), but the prevalence of coronary artery disease was substantially lower (5.1% versus 11.9%)73.
Malnutrition-modulated diabetes mellitus is similar to FCPD, but without the pancreatic calculi74. Its existence was first reported from the eastern parts of India (the states of Orissa and Bihar) but the lack of definitive criteria for its diagnosis makes this a dubious entity. The exact aetiology of this condition remains obscure, although an autoimmune mechanism has been postulated74. The currently accepted classification of diabetes mellitus does not include malnutrition-modulated diabetes mellitus75.
Gestational diabetes mellitus
The prevalence of gestational diabetes mellitus (GDM) in a population is proportional to that of T2DM and impaired glucose tolerance76. Unsurprisingly, therefore, the prevalence of GDM is rapidly increasing in India. Studies in the early 1980s on this condition reported a prevalence of 2% in India77. By 2008, investigators were reporting a prevalence of 17.8% in urban areas, 13.8% in semi-urban areas and 9.9% in rural areas78. Moreover, wide regional variation exists in the prevalence of GDM in India, with the lowest rates (3.8%) being noted in Kashmir79, and the highest (16.2%) in Tamil Nadu78.
Women who have had GDM are at sevenfold higher risk of developing T2DM in the future than those with normal glucose tolerance during pregnancy80. Moreover, T2DM has been suggested to occur more frequently and at a faster rate among Asian Indian people than in other ethnic groups (for example, a 50% conversion rate within 5 years is seen in Asian Indian people, compared with <20% for non-Latin American white individuals)81,82,83. Education regarding diet, physical activity and regular screening can delay or prevent the development of T2DM in these women. Similarly, children born to mothers with GDM are prone to develop obesity and T2DM later in life84. Encouraging the adoption of healthy lifestyle practices in these children can prevent them from developing diabetes mellitus85. Such healthy lifestyle practices are of particular importance in the Asian Indian population, in whom the background risk of T2DM is already high. Prompt diagnosis, appropriate management and close follow-up of women with GDM and their offspring has the potential to prevent diabetes mellitus in two generations and to disrupt the vicious intergenerational cycle of the disease.
Complications in India
The microvascular and macrovascular complications of diabetes mellitus account for most of the morbidity and mortality associated with the disease. While poor glycaemic control and long duration of illness seem to be the most important risk factors for these complications, evidence suggests that ethnic variability in the susceptibility to the complications might also exist.
Microvascular complications
In studies on the prevalence of diabetic retinopathy in India, a lower prevalence compared with western populations has consistently been seen86,87. In the population-based Chennai Urban Rural Epidemiology (CURES) cohort, the prevalence of retinopathy in patients with self-reported diabetes mellitus was 17.6%88, and very similar figures were reported by two other studies from other regions in India87,89. Most studies conducted in western populations have found a retinopathy prevalence of more than 30% in individuals of similar age and duration of disease90. In these three studies the risk factors for developing diabetic retinopathy were duration of diabetes mellitus and poor glycaemic control.
Migrant Asian Indian populations have a higher prevalence of diabetic nephropathy than native populations of the concerned countries91,92,93. For instance, in a multiethnic population in the UK, proteinuria was found in 32% of Asian Indian men and 21% of women with diabetes mellitus compared to 14% and 9% of white men and women respectively94. Similarly, in a cohort study conducted among patients with diabetes mellitus in the Netherlands, individuals of Asian Indian ancestry had 3.9 higher odds of developing albuminuria, and a 1.45 times higher rate of reduction in glomerular filtration rate than individuals of European ancestry93. However, data from India present a mixed picture. The clinic prevalences of microalbuminuria and frank nephropathy (36.3% and 6.9% respectively) are similar to those reported in the migrant studies noted above94,95,96. However, in the population-based CURES cohort a much lower prevalence of frank nephropathy (2.2%) was reported, although the prevalence of microalbuminuria was similar to that found in the other studies97. Notwithstanding these lower prevalence rates, the numbers of individuals reaching end-stage renal disease as a result of diabetic nephropathy is likely to substantially increase in the near future, on account of the sheer number of people with diabetes mellitus in India. That few of these individuals will be able to afford chronic dialysis or kidney replacement, the only two effective modalities of treatment for end-stage renal disease, is of great concern98.
From the available literature, the prevalence of neuropathy varies greatly depending on the definition of neuropathy applied and the diagnostic tests used. For example, in a study from south India, 19.5% of individuals with newly diagnosed T2DM had neuropathy compared with 27.8% of those with known disease99. In another study from northeastern India, the reported prevalence of neuropathy was 29.5% in newly diagnosed patients with T2DM100, while other investigators have reported a prevalence of 29.2% among recently diagnosed patients in Lucknow101. In studies conducted in multiethnic populations in the UK, Asian Indian individuals were found to have significantly lower rates of large-fibre and small-fibre neuropathy compared with individuals of European ethnicity (OR 0.58; 95% CI 0.37–0.92; P <0.02)102. Improved skin microvascularisation has been postulated as the reason for the reduced prevalence of neuropathy102. However, in other studies from the same investigators, these findings have been challenged. Although Asian Indian people tend to have lower rates of peripheral neuropathy than white individuals and individuals of Afro-Caribbean descent, they had 50% higher risk of painful neuropathy symptoms103.
Macrovascular complications
More than 65% of patients with T2DM die of cardiovascular disease; of these, nearly 80% are attributable to coronary artery disease (CAD)104. The susceptibility of Asian Indian individuals to CAD is well known105. Compared with white individuals, CAD tends to develop a decade or two earlier and triple vessel disease is more common; mortality after an acute coronary event is also 40% higher in Asian Indian patients106. The presence of T2DM seems to confer a 3–4 times higher risk of cardiovascular disease to Asian Indian individuals than to their white counterparts, even after adjusting for sex, age, smoking status, hypertension and obesity107. Possible explanations include the atherogenic milieu promoted by high levels of insulin resistance and the high prevalence of 'atherogenic dyslipidaemia' characterized by high levels of triglycerides and small dense LDL cholesterol, and low levels of HDL cholesterol108.
However, few population-based data exist on the prevalence of CAD in India, particularly comparing people with and without T2DM. In the Chennai Urban Population Study, a population-based study conducted in two residential colonies in Chennai, in South India, CAD had a prevalence of 21.4% among individuals with T2DM, compared with 9.1% among those with normal glucose tolerance and 14.9% among those with impaired glucose tolerance109.
Peripheral vascular disease (PVD) is fortunately rare among patients with diabetes mellitus in India. Younger age of onset and relatively low prevalence of smoking are perhaps responsible for the low prevalence of PVD. In the CURES cohort110, the prevalence of PVD was 8.6%, compared with 23.5% among patients with T2DM in the UK111 and 20–30% in the USA112. Increased age, female sex and duration of disease were all related to increased incidence of PVD110.
Diabetic foot ulcers
Diabetic foot ulcers and infections are responsible for >30% of the hospitalisations related to diabetes mellitus113. 25% of people with diabetes mellitus are estimated to develop a foot ulcer during their lifetime114. Diabetic foot ulceration is also an expensive complication of diabetes mellitus, owing to both medical care and on account of time lost from work and loss of income and financial independence115,116. The majority (>80%) of foot ulcers in India arise in neuropathic feet, with only a third having vascular insufficiency117, which, importantly, implies that most of these ulcers can be prevented with proper patient education on appropriate foot care.
Infections
India is facing a double disease burden, with both the persistence of communicable diseases and the emergence of NCDs. Communicable diseases such as typhoid, cholera, malaria and dengue continue to be rampant in many parts of India118, but tuberculosis deserves special mention. Diabetes mellitus and tuberculosis have a bidirectional relationship. Approximately 25% of patients with tuberculosis are estimated to have diabetes mellitus119, and tuberculosis occurs in up to 8% of patients with diabetes mellitus120. Tuberculosis in patients with diabetes mellitus might present with atypical features, such as predominant lower lobe involvement, and thereby delay the diagnosis. Also, cure rates of tuberculosis are lower in patients with diabetes mellitus than those with tuberculosis alone (treatment failure rates 4.2% versus 0.7%)121. Prompt diagnosis and initiation of antituberculous chemotherapy, along with achievement of tight glycaemic control, are essential to ensure cure and prevention of reactivation of tuberculosis.
Diabetes mellitus care in India
The epidemic of diabetes mellitus and associated NCDs threatens to derail much of the progress India has made in health care over the past four decades. In 2013, an average Indian patient with diabetes mellitus was estimated to have spent Indian Rs4,493 (US$95) a year on treatment expenses. The figure increased to Rs12,690 ($270) if renal disease was present and to Rs19,020 ($404) if diabetic foot disease was present122. The magnitude of this expense can be understood if one considers that the per capita income in India during 2013–2014 was only Rs74,920 ($1,570). Owing to low penetrance of health insurance, much of this expenditure is, therefore, borne by the patient.
Diabetes mellitus care in India has a long history. The first diabetes mellitus clinics began in the government sector in the late 1940s in Chennai and Kolkata123; the former continues to function uninterrupted to this day. In the early 1970s, the first private hospital solely devoted to patients with diabetes mellitus was founded. Today, most of the larger cities of the country are served by well-equipped clinics devoted to caring for patients with diabetes mellitus, which are staffed by experienced physicians and paramedical staff124.
Unfortunately, the situation is different in rural areas. Although 75% of India's population lives in rural areas, only 25% of medical practitioners work there124. Specialists in diabetes mellitus (and many other fields) are almost exclusively found in urban areas. Even in urban areas, a shortage exists of trained specialists in diabetes mellitus care, endocrinologists and specialized paramedical staff such as educators, nurses and podiatrists124. The ICMR–INDIAB study assessed knowledge and awareness, using a questionnaire, of diabetes mellitus in four regions of India in individuals with and without the disease. In this study, awareness regarding diabetes mellitus and its complications is low even among individuals who have the disease125. In many parts of the country, particularly in rural areas, the prevalence of undiagnosed diabetes mellitus is high, with nearly three undiagnosed individuals for every known case19. Under these circumstances, that the quality of care for diabetes mellitus in India is patchy is unsurprising. In the population-based ICMR–INDIAB study only approximately a third of individuals with self-reported diabetes mellitus in India had good control of their disease as defined by levels of HbA1c of <7%126. The situation is no better in the clinics; in the DiabCare India 2011 study conducted in 330 centres across, mean HbA1c of the 6,168 patients studied was 8.9%127, a figure unchanged from a decade ago128.
The Government of India, taking cognizance of the growing burden of NCDs, launched the National Programme for Prevention and Control of Cancer, Diabetes, Cardiovascular Diseases & Stroke in 2010 (Ref. 129). This programme aims to prevent and control NCDs through lifestyle changes, provide early diagnosis and treatment and to build capacity and train individuals within the public health setup to cope with the rising burden of NCDs.
However, diabetes mellitus in India is too large a problem for the government to tackle alone. In India, most medical care for this disease is provided by general practitioners in the private sector. Several initiatives have, therefore, been launched under the public–private partnership model to improve the knowledge base of these medical practitioners and thereby build capacity in care to improve the quality of diabetes treatment in India. For example, the Certificate Course in Evidence Based Diabetes Management (CCEBDM) and the Certificate Course in Gestational Diabetes Mellitus (CCGDM) and the newly launched Certified Course in Diabetic Retinopathy conducted in collaboration with the Public Health Foundation of India are three such schemes130,131. CCEBDM has trained >7,500 physicians in the management of diabetes mellitus, while CCGDM has trained >2,500 obstetricians and physicians in the management of GDM. The National Diabetes Educators Program has also trained >2,500 educators to become links between doctors and patients and improve the quality of care132.
The application of new technologies such as telemedicine has the potential to revolutionise care for patients with diabetes mellitus in hitherto underserved rural areas of India. For example, the Chunampet Rural Diabetes Prevention Project was undertaken in a cluster of 42 villages in and around the Chunampet village in the state of Tamil Nadu in southern India133. This population was screened for diabetes mellitus and its complications using a telemedicine approach equipped with retinal photography, Doppler imaging, biothesiometry and electrocardiography. A vehicle equipped with the necessary equipment toured villages in this area to screen and assess any complications at the patient's doorstep. Retinal photographs and other investigation reports were immediately transmitted to the main urban assessment centre via a satellite link. Consultants in the urban centre then assessed the reports and engaged with the patients on a real-time basis via the satellite link134. A rural centre was established to provide basic diabetes mellitus care for those individuals who required care beyond what could be provided by telemedicine alone. Following this intervention, mean HbA1c decreased from 9.3 ± 2.6% to 8.5 ± 2.4% within 1 year and <5% of patients needed referral to tertiary care units134. The Chunampet Rural Diabetes Prevention Project is considered a successful model for screening and delivery of diabetes mellitus care and prevention to rural areas in India and other developing countries135.
However, the high cost of conventional retinal fundus cameras is a major barrier to widespread screening for diabetic retinopathy in many parts of the world. The use of smartphones to diagnose diabetic retinopathy using low-cost technology developed in Bangalore, India, has the potential to make retinal screening accessible, affordable and easily available to people with diabetes mellitus not only in India, but also in other parts of the developing world136. Smartphone-based retinal screening using a fundus-on-phone camera is similar to conventional retinal photography in terms of sensitivity and specificity136.
Prevention
In several randomized controlled trials, T2DM can be prevented in individuals at high risk of developing the disease using lifestyle modification, drugs or a combination of the two137,138,139. However, most of these studies have been performed in developed nations such as the USA and Finland where sufficient resources can translate these findings into a real-life setting136,137. Moreover, and as noted earlier in the text, the nature of T2DM seems to be different in Asian Indian individuals, suggesting that such treatments might not be strictly applicable to this population. The need for prevention of T2DM in India has also been elegantly summarized in another review140.
The investigators of a population-based cohort study have attempted to assess the population attributable risk of diabetes mellitus contributed by common modifiable risk factors in a south Indian population139. In that study, 80% of incident T2DM could be prevented by favourably modifying five risk factors — unhealthy diet, physical inactivity, abdominal obesity, high levels of triglycerides and low levels of HDL cholesterol141. In the Indian Diabetes Prevention Programme, patients with prediabetes were randomly assigned to receive intensive lifestyle modification, metformin or routine care142. At the end of a median follow-up period of 30 months, lifestyle modification was found to reduce the risk of T2DM by 28.5% (compared with 58% in the Diabetes Prevention Program in the USA) and metformin by 26.4% (versus 31%)142. Cost-effectiveness to prevent one case of T2DM with lifestyle modification was Rs47,341 ($1,052), with metformin, Rs49,280 ($1,095), and with the combined intervention, Rs61,133 ($1,359)143. Investigators in the ongoing Diabetes–a Community Lifestyle Intervention Programme are assessing the feasibility and effectiveness of a culturally tailored and appropriate lifestyle intervention programme in preventing T2DM in individuals with prediabetes144.
An interesting preventive approach is the use of mobile-phone based messaging to enable lifestyle changes that might help prevent the development of T2DM. This intervention makes use of the high penetrance of mobile phones (nearly 80% overall, and close to 50% in rural areas)145 in India and seems to be a promising approach for the future146. In an urban population in south India, provision of lifestyle advice through frequent mobile phone messages was associated with a significant reduction in incident T2DM (HR 0.64, 95% CI 0.45–0.92; P = 0.015) over 3 years, compared with control individuals who received only standard lifestyle advice at the baseline visit146.
Ultimately, prevention of T2DM needs to be multisectoral and depends on the involvement and collaboration of many stakeholders such as patients, family, health-care providers and government agencies. Governmental policy decisions that might have an effect on minimizing the risk of developing both T2DM and other metabolic NCDs include: provision of adequate facilities for recreational physical activity; encouraging use of walking or cycling rather than mechanized transport; as well as discouraging consumption of junk food through taxation. Moreover, governments can help ensure the availability of healthy foods such as fresh fruit and vegetables at affordable prices and encourage healthy eating practices in schools. In combination with efforts at awareness creation, these steps will ensure adoption of healthier lifestyles by a wider section of the population and significantly reduce the risk of development of T2DM.
Relevance of the Indian experience
India is the prototypical underdeveloped nation that has undergone a dramatic socioeconomic and demographic transition in the past couple of decades. The far-reaching effects of industrialization and urbanization have combined with an increasingly ageing population to create a fertile milieu for the development of chronic NCDs such as diabetes mellitus. At the same time, communicable diseases continue to contribute to the overall morbidity and mortality and compete with NCDs for the allocation of scarce health-care resources. This situation is prevalent not only in India, but in other emerging economies of Asia, Latin America and Africa147,148. Lessons learned from the history of the diabetes mellitus epidemic in India can, therefore, be used to beneficial effect in these countries as well. Experience gained in managing the disease and its complications in India is also likely to be of immense use to policy makers and health-care providers in countries with large populations of Asian Indian extraction. The salient points learned from the Indian experience with diabetes mellitus over the past four decades are summarized in Box 1.
Conclusions
Hundreds of millions of individuals in Sub-Saharan Africa, Asia and South America are yet to experience the epidemiological transition from communicable diseases to NCD such as diabetes mellitus. For these populations, the lessons learnt from India's experiences in the fight against diabetes mellitus could be applied with local cultural adaptations. The knowledge we possess, if used appropriately with proper community empowerment, has the potential to slow the epidemic of diabetes mellitus. The dividend, in the form of improved health, productivity and economic development, is well worth the effort.
References
International Diabetes Federation. IDF Diabetes Atlas, seventh edition 2015. [online], (2015).
Zimmet, P. Z. Kelly West Lecture 1991. Challenges in diabetes epidemiology — from West to the rest. Diabetes Care 15, 232–252 (1992).
Dowse, G. K. et al. High prevalence of NIDDM and impaired glucose tolerance in Indian, Creole, and Chinese Mauritians. Diabetes 39, 390–396 (1990).
Zimmet, P. et al. Prevalence of diabetes and impaired glucose tolerance in the biracial (Melanesian and Indian) population of Fiji: a rural–urban comparison. Am. J. Epidemiol. 118, 673–688 (1983).
United Nations Geographic Region Classification. List of country names. [online], (2016).
Unnikrishnan, R., Anjana, R. M. & Mohan, V. Diabetes in South Asians: is the phenotype different? Diabetes 63, 53–55 (2014).
Patel, J. C. et al. A sample survey to determine the incidence of diabetes in bombay. J. Indian Med. Assoc. 41, 448–452 (1963).
The K.E.M. Hospital Group. in Diabetes in the Tropics (eds Patel, J. C. & Talwalker, N. G.) 1–79 (Diabetic Association of India, 1966).
Berry, J. N., Chakravarty, R. N., Gupta, H. D. & Malik, K. Prevalence of diabetes mellitus in a north Indian town. Indian J. Med. Res. 54, 1025–1047 (1966).
Gour, K. N. in Diabetes in the Tropics (eds Patel, J. C. & Talwalker, N. G.) 76–79 (Diabetic Association of India, 1966).
Rao, P. S. et al. in Diabetes in the Tropics (eds Patel, J. C. & Talwalker, N. G.) 68–75 (Diabetic Association of India, 1966).
Viswanathan, M., Moses, S. G. P. & Krishnamoorty, M. in Diabetes in the Tropics (eds Patel, J. C., & Talwalker, N. G.) 29–32 (Diabetic Association of India, 1966).
Mohan, V. et al. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban south India — the Chennai Urban Rural Epidemiology Study (CURES-17). Diabetologia 49, 1175–1178 (2006).
Ahuja, M. M. S. (ed.) in Epidemiological Studies on Diabetes Mellitus in India in Epidemiology of Diabetes in Developing Countries 29–38 (Interprint, 1979).
Ramachandran, A. et al. High prevalence of diabetes and impaired glucose tolerance in India: National Urban Diabetes Survey. Diabetologia 44, 1094–1101 (2001).
Sadikot, S. M. et al. The burden of diabetes and impaired fasting glucose in India using the ADA 1997 criteria: Prevalence of Diabetes in India Study (PODIS). Diabetes Res. Clin. Pract. 66, 293–300 (2004).
Anjana, R. M. et al. The need for obtaining accurate nationwide estimates of diabetes prevalence in India — rationale for a national study on diabetes. Indian J. Med. Res. 133, 369–380 (2011).
Anjana, R. M. et al. The Indian Council of Medical Research-India Diabetes (ICMR-INDIAB) study: methodological details. J. Diabetes Sci. Technol. 5, 906–914 (2011).
Anjana, R. M. et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetologia 54, 3022–3027 (2011).
International Diabetes Federation. Diabetes Atlas 5th edn (International Diabetes Federation, 2011).
Beckles, G. L. et al. High total and cardiovascular disease mortality in adults of Indian descent in Trinidad, unexplained by major coronary risk factors. Lancet 1, 1293–1301 (1986).
Simmons, D., Williams, D. R. & Powell, M. J. Prevalence of diabetes in a predominantly Asian community: preliminary findings of the Coventry diabetes study. BMJ 298, 18–21 (1989).
Omar, M. A. K. & Motala, A. A. Diabetes in South African Indians. Int. J. Diabetes Dev. Ctries 16, 45–47 (1996).
Oza-Frank, R., Ali, M. K., Vaccarino, V. & Narayan, K. M. Asian Americans: diabetes prevalence across U.S. and World Health Organization weight classifications. Diabetes Care 32, 1644–1646 (2009).
Oza-Frank, R. & Narayan, K. M. Overweight and diabetes prevalence among US immigrants. Am. J. Publ. Health 100, 661–668 (2010).
National Registry of Diseases Office. Information paper on diabetes in Singapore. [online], (2011).
Wan Nazaimoon, W. M. et al. Prevalence of diabetes in Malaysia and usefulness of HbA1c as a diagnostic criterion. Diabet. Med. 30, 825–828 (2013).
Lin, S. et al. Diabetes and obesity trends in Fiji over 30 years. J. Diabetes http://dx.doi.org/10.1111/1753-0407.12326 (2015).
Anjana, R. M. et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES). Diabetes Care 38, 1441–1448 (2015).
Diamond, J. Medicine: diabetes in India. Nature 469, 478–479 (2011).
Qiao, Q. et al. Age- and sex-specific prevalence of diabetes and impaired glucose regulation in 11 Asian cohorts. Diabetes Care 26, 1770–1780 (2003).
McKeigue, P. M., Shah, B. & Marmot, M. G. Relation of central obesity and insulin resistance with high diabetes prevalence and cardiovascular risk in South Asians. Lancet 337, 382–386 (1991).
Pandit, K., Goswami, S., Ghosh, S., Mukhopadhyay, P. & Chowdhury, S. Metabolic syndrome in South Asians. Indian J. Endocrinol. Metab. 16, 44–55 (2012).
Misra, A. & Khurana, L. The metabolic syndrome in South Asians: epidemiology, determinants, and prevention. Metab. Syndr. Relat. Disord. 7, 497–514 (2009).
Mohan, V. et al. Serum immunoreactive insulin responses to a glucose load in Asian Indian and European type 2 (non-insulin-dependent) diabetic patients and control subjects. Diabetologia 29, 235–237 (1986).
Misra, A. et al. High prevalence of insulin resistance in postpubertal Asian Indian children is associated with adverse truncal body fat patterning, abdominal adiposity and excess body fat. Int. J. Obes. Relat. Metab. Disord. 28, 1217–1226 (2004).
Yajnik, C. S. et al. Adiposity and hyperinsulinemia in Indians are present at birth. J. Clin. Endocrinol. Metab. 87, 5575–5580 (2002).
Ikehara, S. et al. Age trajectories of glycaemic traits in non-diabetic South Asian and white individuals: the Whitehall II cohort study. Diabetologia 58, 534–542 (2015).
Staimez, L. R. et al. Evidence of reduced β-cell function in Asian Indians with mild dysglycemia. Diabetes Care 36, 2772–2778 (2013).
Kooner, J. S. et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat. Genet. 43, 984–989 (2011).
Tabassum, R. et al. Genome-wide association study for type 2 diabetes in Indians identifies a new susceptibility locus at 2q21. Diabetes 62, 977–986 (2013).
Saxena, R. et al. Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in Sikhs of Punjabi origin from India. Diabetes 62, 1746–1755 (2013).
Radha, V. et al. Role of genetic polymorphism peroxisome proliferator-activated receptor-γ2 Pro12Ala on ethnic susceptibility to diabetes in South-Asian and Caucasian subjects: evidence for heterogeneity. Diabetes Care 29, 1046–1051 (2006).
Yajnik, C. S. & Deshmukh, U. S. Maternal nutrition, intrauterine programming and consequential risks in the offspring. Rev. Endocr. Metab. Disord. 9, 203–211 (2008).
Yajnik, C. S. et al. Vitamin B12 and folate concentrations during pregnancy and insulin resistance in the offspring: the Pune Maternal Nutrition Study. Diabetologia 51, 29–38 (2008).
Vickers, M. H., Reddy, S., Ikenasio, B. A. & Breier, B. H. Dysregulation of the adipoinsular axis — a mechanism for the pathogenesis of hyperleptinemia and adipogenic diabetes induced by fetal programming. J. Endocrinol. 170, 323–332 (2001).
Bhargava, S. K. et al. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N. Engl. J. Med. 350, 865–875 (2004).
Ng, S. W. & Popkin, B. M. Time use and physical activity: a shift away from movement across the globe. Obes. Rev. 13, 659–680 (2012).
Anjana, R. M. et al. Physical activity and inactivity patterns in India — results from the ICMR-INDIAB study (Phase-1) [ICMR-INDIAB-5]. Int. J. Behav. Nutr. Phys. Act 11, 26 (2014).
Mohan, V., Radhika, G., Vijayalakshmi, P. & Sudha, V. Can the diabetes/cardiovascular disease epidemic in India be explained, at least in part, by excess refined grain (rice) intake? Indian J. Med. Res. 131, 369–372 (2010).
Radhika, G., Van Dam, R. M., Sudha, V., Ganesan, A. & Mohan, V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57). Metabolism 58, 675–681 (2009).
Ebrahim, S. et al. The effect of rural-to-urban migration on obesity and diabetes in India: a cross-sectional study. PLoS Med. 7, e1000268 (2010).
Pradeepa, R. et al. Prevalence of generalized & abdominal obesity in urban & rural India — the ICMR-INDIAB Study (Phase-I) [ICMR - INDIAB-3]. Indian J. Med. Res. 142, 139–150 (2015).
Bhardwaj, S. et al. High prevalence of abdominal, intra-abdominal and subcutaneous adiposity and clustering of risk factors among urban Asian Indians in North India. PLoS ONE 6, e24362 (2011).
Misra, A. et al. The high burden of obesity and abdominal obesity in urban Indian schoolchildren: a multicentric study of 38,296 children. Ann. Nutr. Metab. 58, 203–211 (2011).
Gujral, U. P. et al. Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U.S.: The CARRS and MASALA Studies. Diabetes Care 38, 1312–1318 (2015).
Deepa, M., Anjana, R. M., Manjula, D., Narayan, K. M. & Mohan, V. Convergence of prevalence rates of diabetes and cardiometabolic risk factors in middle and low income groups in urban India: 10-year follow-up of the Chennai Urban Population Study. J. Diabetes Sci. Technol. 5, 918–927 (2011).
Misra, A. et al. Insulin resistance and clustering of atherogenic risk factors in women belonging to low socio-economic strata in urban slums of North India. Diabetes Res. Clin. Pract. 56, 73–75 (2002).
Kumar, K. M., Azad, K., Zabeen, B. & Kalra, S. Type 1 diabetes in children: fighting for a place under the sun. Indian J. Endocrinol. Metab. 16, S1–S3 (2012).
Ramachandran, A., Snehalatha, C. & Krishnaswamy, C. V. Incidence of IDDM in children in urban population in Southern India. Diabetes Res. Clin. Pract. 34, 79–82 (1996).
Kumar, P. et al. Incidence of type 1 diabetes mellitus and associated complications among children and young adults: results from Karnataka Diabetes Registry 1995–2008. J. Indian Med. Assoc. 106, 708–711 (2008).
Kalra, S., Kalra, B. & Sharma, A. Prevalence of type 1 diabetes mellitus in Karnal district, Haryana state, India. Diabetol. Metab. Syndr. 2, 14 (2010).
Karvonen, M. et al. Incidence of childhood type 1 diabetes worldwide. Diabetes Care 23, 1516–1526 (2000).
Balasubramanian, K. et al. High frequency of type 1B (idiopathic) diabetes in North Indian children with recent-onset diabetes. Diabetes Care 26, 2697 (2003).
Kanga, U., Vaidyanathan, B., Jaini, R., Menon, P. S. & Mehra, N. K. HLA haplotypes associated with type 1 diabetes mellitus in North Indian children. Hum. Immunol. 65, 47–53 (2004).
Tattersall, R. B. & Fajans, S. S. A difference between the inheritance of classical juvenile-onset and maturity-onset type diabetes of young people. Diabetes 24, 44–53 (1975).
Radha, V. et al. Identification of novel variants in the hepatocyte nuclear factor-1α gene in South Indian patients with maturity onset diabetes of young. J. Clin. Endocrinol. Metab. 94, 1959–1965 (2009).
Anuradha, S., Radha, V. & Mohan, V. Association of novel variants in the hepatocyte nuclear factor 4A gene with maturity onset diabetes of the young and early onset type 2 diabetes. Clin. Genet. 80, 541–549 (2011).
Chapla, A. et al. Maturity onset diabetes of the young in India — a distinctive mutation pattern identified through targeted next-generation sequencing. Clin. Endocrinol. (Oxf.) 82, 533–542 (2015).
Unnikrishnan, R. & Mohan, V. Fibrocalculous pancreatic diabetes (FCPD). Acta Diabetol. 52, 1–9 (2015).
Mohan, V., Farooq, S. & Deepa, M. Prevalence of fibrocalculous pancreatic diabetes in Chennai in South India. JOP 9, 489–492 (2008).
Papita, R. et al. Secular trends of fibrocalculous pancreatic diabetes and diabetes secondary to alcoholic chronic pancreatitis at a tertiary care diabetes centre in South India. JOP 13, 205–209 (2012).
Kanta Barman, K. et al. Prevalence of diabetic complications in fibrocalculous pancreatic diabetic patients and type 2 diabetic patients: a cross-sectional comparative study. J. Diabetes Complications 18, 264–270 (2004).
Kanungo, A., Samal, K. C. & Sanjeevi, C. B. Molecular mechanisms involved in the etiopathogenesis of malnutrition-modulated diabetes mellitus. Ann. NY Acad. Sci. 958, 138–143 (2002).
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 39, S13–S22 (2016).
American College of Obstetricians and Gynecologists. Practice bulletin, clinical management guidelines for obstetrician–gynecologists. [online], (2001)
Agarwal, S. & Gupta, A. N. Gestational diabetes. J. Assoc. Physicians India 30, 203–205 (1982).
Seshiah, V. et al. Prevalence of gestational diabetes mellitus in South India (Tamil Nadu) — a community based study. J Assoc. Physicians India 56, 329–333 (2008).
Zargar, A. H. et al. Prevalence of gestational diabetes mellitus in Kashmiri women from the Indian subcontinent. Diabetes Res. Clin. Pract. 66, 139–145 (2004).
Buchanan, T. A. & Xiang, A. H. Gestational diabetes mellitus. J. Clin. Invest. 115, 485–491 (2005).
Kale, S. D. et al. High risk of diabetes and metabolic syndrome in Indian women with gestational diabetes mellitus. Diabet. Med. 21, 1257–1258 (2004).
Kim, C., Newton, K. M. & Knopp, R. H. Gestational diabetes and the incidence of type 2 diabetes: a systematic review. Diabetes Care 25, 1862–1868 (2002).
Mahalakshmi, M. M. et al. Clinical profile, outcomes, and progression to type 2 diabetes among Indian women with gestational diabetes mellitus seen at a diabetes center in south India. Indian J. Endocrinol. Metab. 18, 400–406 (2014).
Dabelea, D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care 30, S169–S174 (2007).
Eriksson, J., Lindström, J. & Tuomilehto, J. Potential for the prevention of type 2 diabetes. Br. Med. Bull. 60, 183–199 (2001).
Dandona, L., Dandona, R., Shamanna, B. R., Naduvilath, T. J. & Rao, G. N. Developing a model to reduce blindness in India: the International Centre for Advancement of Rural Eye Care. Indian J. Ophthalmol. 46, 263–268 (1998).
Narendran, V. et al. Diabetic retinopathy among self reported diabetics in southern India: a population based assessment. Br. J. Ophthalmol. 86, 1014–1018 (2002).
Rema, M. et al. Prevalence of diabetic retinopathy in urban India: the Chennai Urban Rural Epidemiology Study (CURES) eye study, I. Invest. Ophthalmol. Vis. Sci. 46, 2328–2333 (2005).
Raman, R. et al. Prevalence of diabetic retinopathy in India: Sankara Nethralaya Diabetic Retinopathy Epidemiology and Molecular Genetics Study report 2. Ophthalmology 116, 311–318 (2009).
Rema, M. & Pradeepa, R. Diabetic retinopathy: an Indian perspective. Indian J. Med. Res. 125, 297–310 (2007).
Samanta, A., Burden, A. C., Feehally, J. & Walls, J. Diabetic renal disease: differences between Asian and white patients. Br. Med. J. (Clin. Res. Ed.) 293, 366–367 (1986).
Mather, H. M., Chaturvedi, N. & Kehely, A. M. Comparison of prevalence and risk factors for microalbuminuria in South Asians and Europeans with type 2 diabetes mellitus. Diabet. Med. 15, 672–677 (1998).
Chandie Shaw, P. K. et al. South-Asian type 2 diabetic patients have higher incidence and faster progression of renal disease compared with Dutch-European diabetic patients. Diabetes Care 29, 1383–1385 (2006).
Vijay, V., Snehalatha, C., Ramachandran, A. & Viswanathan, M. Prevalence of proteinuria in non-insulin dependent diabetes. J. Assoc. Physicians India 42, 792–794 (1994).
Mohan, V. et al. Frequency of proteinuria in type 2 diabetes mellitus seen at a diabetes centre in southern India. Postgrad. Med. J. 76, 569–573 (2000).
Varghese, A., Deepa, R., Rema, M. & Mohan, V. Prevalence of microalbuminuria in type 2 diabetes mellitus at a diabetes centre in southern India. Postgrad. Med. J. 77, 399–402 (2001).
Unnikrishnan, R. I. et al. Prevalence and risk factors of diabetic nephropathy in an urban South Indian population: the Chennai Urban Rural Epidemiology Study (CURES 45). Diabetes Care 30, 2019–2024 (2007).
Mani, M. K. Treating renal disease in India's poor: the art of the possible. Semin. Nephrol. 30, 74–80 (2010).
Pradeepa, R. et al. Prevalence and risk factors for diabetic neuropathy in an urban south Indian population: the Chennai Urban Rural Epidemiology Study (CURES-55). Diabet. Med. 25, 407–412 (2008).
Dutta, A., Naorem, S., Singh, P. & Wangjam, K. Prevalence of peripheral neuropathy in newly diagnosed type 2 diabetics. Int. J. Diabetes Dev. Ctries 25, 30–33 (2005).
Gill, H. K., Yadav, S. B., Ramesh, V. & Bhatia, E. A prospective study of prevalence and association of peripheral neuropathy in Indian patients with newly diagnosed type 2 diabetes mellitus. J. Postgrad. Med. 60, 270–275 (2014).
Abbott, C. A. et al. Explanations for the lower rates of diabetic neuropathy in Indian Asians versus Europeans. Diabetes Care 33, 1325–1330 (2010).
Abbott, C. A., Malik, R. A., van Ross, E. R., Kulkarni, J. & Boulton, A. J. Prevalence and characteristics of painful diabetic neuropathy in a large community-based diabetic population in the U.K. Diabetes Care 34, 2220–2224 (2011).
Ali, M. K., Narayan, K. M. & Tandon, N. Diabetes and coronary heart disease: current perspectives. Indian J. Med. Res. 132, 584–597 (2010).
Enas, E. A. et al. Reducing the burden of coronary artery disease in India: challenges and opportunities. Indian Heart J. 60, 161–175 (2008).
Mohan, V., Venkatraman, J. V. & Pradeepa, R. Epidemiology of cardiovascular disease in type 2 diabetes: the Indian scenario. J. Diabetes Sci. Technol. 4, 158–170 (2010).
Forouhi, N. G., Sattar, N., Tillin, T., McKeigue, P. M. & Chaturvedi, N. Do known risk factors explain the higher coronary heart disease mortality in South Asian compared with European men? Prospective follow-up of the Southall and Brent studies, UK. Diabetologia 49, 2580–2588 (2006).
Enas, E. A. et al. The metabolic syndrome and dyslipidemia among Asian Indians: a population with high rates of diabetes and premature coronary artery disease. J. Cardiometab. Syndr. 2, 267–275 (2007).
Mohan, V., Deepa, R., Rani, S. S. & Premalatha, G. Prevalence of coronary artery disease and its relationship to lipids in a selected population in South India: The Chennai Urban Population Study (CUPS No. 5). J. Am. Coll. Cardiol. 38, 682–687 (2001).
Pradeepa, R. et al. Prevalence of peripheral vascular disease and its association with carotid intima-media thickness and arterial stiffness in type 2 diabetes: the Chennai urban rural epidemiology study (CURES 111). Diab. Vasc. Dis. Res. 11, 190–200 (2014).
Walters, D. P., Gatling, W., Mullee, M. A. & Hill, R. D. The prevalence, detection, and epidemiological correlates of peripheral vascular disease: a comparison of diabetic and non-diabetic subjects in an English community. Diabet. Med. 9, 710–715 (1992).
Marso, S. P. & Hiatt, W. R. Peripheral arterial disease in patients with diabetes. J. Am. Coll. Cardiol. 47, 921–929 (2006).
Gupta, S. in Medicine Update 2012 (ed. Kamath, S.) 287–293 (Mumbai, 2012).
Singh, N., Armstrong, D. G. & Lipsky, B. A. Preventing foot ulcers in patients with diabetes. JAMA 293, 217–228 (2005).
Pendsey, S. P. Epidemiological aspects of diabetes foot. Int. J. Diabetes Dev. Ctries 14, 37–38 (1994).
Cavanagh, P. et al. Cost of treating diabetic foot ulcers in five different countries. Diabetes Metab. Res. Rev. 28 (Suppl. 1), 107–111 (2012).
Viswanathan, V. Epidemiology of diabetic foot and management of foot problems in India. Int. J. Low. Extrem. Wounds 9, 122–126 (2010).
Dikid, T., Jain, S. K., Sharma, A., Kumar, A. & Narain, J. P. Emerging and re-emerging infections in India: an overview. Indian J. Med. Res. 138, 19–31 (2013).
Viswanathan, V. et al. Prevalence of diabetes and pre-diabetes and associated risk factors among tuberculosis patients in India. PLoS ONE 7, e41367 (2012).
Gupta, A. & Shah, A. Tuberculosis and diabetes: appraisal. Indian J. Tuberc. 47, 3–8 (2000).
Viswanathan, V. et al. Effect of diabetes on treatment outcome of smear-positive pulmonary tuberculosis — a report from South India. J. Diabetes Complications 28, 162–165 (2014).
Kumpatla, S., Kothandan, H., Tharkar, S. & Viswanathan, V. The costs of treating long-term diabetic complications in a developing country: a study from India. J. Assoc. Physicians India 61, 102–109 (2013).
Research Society for the Study of Diabetes in India. Obituary — Prof. M. Viswanathan. Int. J. Diabetes Dev. Ctries 16, 67 (1996).
Joshi, S. R., Das, A. K., Vijay, V. J. & Mohan, V. Challenges in diabetes care in India: sheer numbers, lack of awareness and inadequate control. J. Assoc. Physicians India 56, 443–450 (2008).
Deepa, M. et al. Knowledge and awareness of diabetes in urban and rural India: The Indian Council of Medical Research India Diabetes Study (Phase I): Indian Council of Medical Research India Diabetes 4. Indian J. Endocrinol. Metab. 18, 379–385 (2014).
Unnikrishnan, R. et al. Glycemic control among individuals with self-reported diabetes in India — the ICMR-INDIAB Study. Diabetes Technol. Ther. 16, 596–603 (2014).
Mohan, V. et al. Current status of management, control, complications and psychosocial aspects of patients with diabetes in India: results from the DiabCare India 2011 Study. Indian J. Endocrinol. Metab. 18, 370–378 (2014).
Raheja, B. S. et al. DiabCare Asia — India Study: diabetes care in India — current status. J. Assoc. Physicians India 49, 717–722 (2001).
Government of India. Ministry of Health and Family Welfare. National Programme For Prevention and Control of Cancer, Diabetes, Cardiovascular Disease and Stroke (NPCDCS). [online], (2016).
Public Health Foundation of India. The Certificate Course in Evidence Based Diabetes Management (CCEBDM). [online], (2015).
Public Health Foundation of India. The Certificate Course in Gestational Diabetes Mellitus (CCGDM). [online], (2015).
Joshi, S., Joshi, S. R. & Mohan, V. Methodology and feasibility of a structured education program for diabetes education in India: The National Diabetes Educator Program. Indian J. Endocrinol. Metab. 17, 396–401 (2013).
Mohan, V. et al. Prevention of diabetes in rural India with a telemedicine intervention. J. Diabetes Sci. Technol. 6, 1355–1364 (2012).
Mohan, V., Prathiba, V. & Pradeepa, R. Tele-diabetology to screen for diabetes and associated complications in rural India: The Chunampet Rural Diabetes Prevention Project Model. J. Diabetes Sci. Technol. 8, 256–261 (2014).
Patel, V. et al. India: towards universal health coverage 3 — chronic diseases and injuries in India. Lancet 377, 413–428 (2011).
Rajalakshmi, R. et al. Validation of smartphone based retinal photography for diabetic retinopathy screening. PLoS ONE 10, e0138285 (2015).
Li, G. et al. Effects of insulin resistance and insulin secretion on the efficacy of interventions to retard development of type 2 diabetes mellitus: the DA Qing IGT and Diabetes Study. Diabetes Res. Clin. Pract. 58, 193–200 (2002).
Knowler, W. C. et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N. Engl. J. Med. 346, 393–403 (2002).
Tuomilehto, J. et al. Prevention of Type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N. Engl. J. Med. 344, 1343–1350 (2001).
Misra, A. Prevention of type 2 diabetes: the long and winding road. Lancet 374, 1655–1656 (2009).
Anjana, R. M. et al. Diabetes in Asian Indians — how much is preventable? Ten-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES-142). Diabetes Res. Clin. Pract. 109, 253–261 (2015).
Ramachandran, A. et al. The Indian Diabetes Prevention Programme shows that lifestyle modification and metformin prevent type 2 diabetes in Asian Indian subjects with impaired glucose tolerance (IDPP-1). Diabetologia 49, 289–297 (2006).
Ramachandran, A., Snehalatha, C., Yamuna, A., Mary, S. & Ping, Z. Cost-effectiveness of the interventions in the primary prevention of diabetes among Asian Indians: within-trial results of the Indian Diabetes Prevention Programme (IDPP). Diabetes Care 30, 2548–2552 (2007).
Weber, M. B., Ranjani, H., Meyers, G. C., Mohan, V. & Narayan, K. M. A model of translational research for diabetes prevention in low and middle-income countries: the Diabetes Community Lifestyle Improvement Program (D-CLIP) trial. Prim. Care Diabetes 6, 3–9 (2012).
Telecom Regulatory Authority of India. Highlights of telecome subscription data as of 30th November 2015. [online], (2016).
Ramachandran, A. et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomized controlled trial. Lancet Diabetes Endocrinol. 1, 191–198 (2013).
Gill, G. V., Mbanya, J. C., Ramaiya, K. L. & Tesfaye, S. A Sub-Saharan African perspective of diabetes. Diabetologia 52, 8–16 (2009).
Arredondo, A. Type 2 diabetes and health care costs in Latin America: exploring the need for greater preventive medicine. BMC Med. 12, 136 (2014).
Ravikumar, P. et al. Prevalence and risk factors of diabetes in a community-based study in North India: the Chandigarh Urban Diabetes Study (CUDS). Diabetes Metab. 37, 216–221 (2011).
Gupta, R. et al. Persistent high prevalence of cardiovascular risk factors in the urban middle class in India: Jaipur Heart Watch-5. J. Assoc. Physicians India 60, 11–16 (2012).
Vijayakumar, G., Arun, R. & Kutty, V. R. High prevalence of type 2 diabetes mellitus and other metabolic disorders in rural central Kerala. J. Assoc. Physicians India 57, 563–567 (2009).
Rao, C. R., Kamath, V. G., Shetty, A. & Kamath, A. A study on the prevalence of type 2 diabetes in coastal Karnataka. Int. J. Diabetes Dev. Ctries 30, 80–85 (2010).
Muninarayana, C., Balachandra, G., Hiremath, S. G., Iyengar, K. & Anil, N. S. Prevalence and awareness regarding diabetes mellitus in rural Tamaka, Kolar. Int. J. Diabetes Dev. Ctries 30, 18–21 (2010).
Bharati, D. R. et al. Prevalence and determinants of diabetes mellitus in Puducherry, South India. J. Pharm. Bioallied Sci. 3, 513–518 (2011).
Murthy, P. D., Prasad, K. T., Gopal, P. V., Rao, K. V. & Rao, R. M. A survey for prevalence of coronary artery disease and its risk factors in an urban population in Andhra Pradesh. J. Assoc. Physicians India 60, 17–20 (2012).
Gujral, U. P. et al. Comparing type 2 diabetes, prediabetes, and their associated risk factors in Asian Indians in India and in the U. S.: the CARRS and MASALA studies. Diabetes Care 38, 1312–1318 (2015).
Kumar, S. et al. Prevalence of diabetes and impaired fasting glucose in a selected population with special reference to influence of family history and anthropometric measurements — the Kolkata Policeman Study. J. Assoc. Physicians India 56, 841–844 (2008).
Shah, A. & Afzal, M. Prevalence of diabetes and hypertension and association with various risk factors among different Muslim populations of Manipur. Indian J. Diabetes Metab. Disord. 12, 52 (2013).
Kumar, P. et al. Prevalence of diabetes mellitus, impaired fasting glucose, impaired glucose tolerance, and its correlates among police personnel in Bankura District of West Bengal. Indian J. Publ. Health 57, 24–28 (2013).
Zaman, F. A. & Borang, A. Prevalence of diabetes mellitus amongst rural hilly population of North Eastern India and its relationship with associated risk factors and related co-morbidities. J. Nat. Sci. Biol. Med. 5, 383–388 (2014).
Vaz, N. C., Ferreira, A. M., Kulkarni, M. S. & Vaz, F. S. Prevalence of diabetes mellitus in a rural population of Goa, India. Natl Med. J. India 24, 16–18 (2011).
Author information
Authors and Affiliations
Contributions
R.U. wrote the manuscript. All authors contributed equally to researching data for the article, reviewing and editing the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Information
Supplementary Information S1 (figure) (PDF 2100 kb)
Rights and permissions
About this article
Cite this article
Unnikrishnan, R., Anjana, R. & Mohan, V. Diabetes mellitus and its complications in India. Nat Rev Endocrinol 12, 357–370 (2016). https://doi.org/10.1038/nrendo.2016.53
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.53
This article is cited by
-
The associations of risk of cardiovascular disease with development stages of diabetes in Chinese population: findings from a retrospective cohort study in QuZhou city
BMC Endocrine Disorders (2024)
-
Cost of diabetes and its complications: results from a STEPS survey in Punjab, India
Global Health Research and Policy (2023)
-
Risk of cataract and glaucoma among older persons with diabetes in India: a cross-sectional study based on LASI, Wave-1
Scientific Reports (2023)
-
Expert Consensus Recommendations on Time in Range for Monitoring Glucose Levels in People with Diabetes: An Indian Perspective
Diabetes Therapy (2023)
-
Genetics of Diabetic Kidney Disease in Type 2 Diabetes: Candidate Gene Studies and Genome-Wide Association Studies (GWAS)
Journal of the Indian Institute of Science (2023)