Key Points
-
In general, an inverse relationship between smoking and BMI exists
-
Post-cessation-related obesity might contribute to insulin resistance
-
The number one reason for not wanting to quit smoking or quitting and then relapsing is fear of post-cessation weight gain, especially in women and in individuals with obesity
-
Future smoking cessation programs and therapies need to be designed with an emphasis on reducing post-cessation weight gain
-
The benefits of smoking cessation outweigh the risks
Abstract
Smoking continues to be the leading cause of preventable death in the USA, despite the vast and widely publicized knowledge about the negative health effects of tobacco smoking. Data show that smoking cessation is often accompanied by weight gain and an improvement in insulin sensitivity over time. However, paradoxically, post-cessation-related obesity might contribute to insulin resistance. Furthermore, post-cessation weight gain is reportedly the number one reason why smokers, especially women, fail to initiate smoking cessation or relapse after initiating smoking cessation. In this Review, we discuss the metabolic effects of stopping smoking and highlight future considerations for smoking cessation programs and therapies to be designed with an emphasis on reducing post-cessation weight gain.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Change history
30 September 2016
On page 302 of the above article, VLDL in Figure 2 was incorrectly labelled as VDL. This has been corrected online.
References
World Health Organization. Tobacco. Fact sheet N°339. [online], (2015).
Yuen, B. G. et al. Association between smoking and uveitis: results from the Pacific Ocular Inflammation Study. Ophthalmology 122, 1257–1261 (2015).
Roura, E. et al. Smoking as a major risk factor for cervical cancer and pre-cancer: results from the EPIC cohort. Int. J. Cancer 135, 453–466 (2014).
Jaramillo, J. D. et al. Reduced bone density and vertebral fractures in smokers. Men and COPD patients at increased risk. Ann. Am. Thorac. Soc. 12, 648–656 (2015).
US Department of Health and Human Services. The health consequences of smoking—50 years of progress: a report of the Surgeon General, 2014 [online], (2014).
Practice Committee of the American Society for Reproductive Medicine. Smoking and infertility: a committee opinion. Fertil. Steril. 98, 1400–1406 (2012).
Ruan, X. & Mueck, A. O. Impact of smoking on estrogenic efficacy. Climacteric 18, 38–46 (2015).
Pedone, C. & Incalzi, R. A. Smoking and mortality — beyond established causes. N. Engl. J. Med. 372, 2169 (2015).
Grando, S. A. Connections of nicotine to cancer. Nat. Rev. Cancer 14, 419–429 (2014).
Kowall, B. et al. Association of passive and active smoking with incident type 2 diabetes mellitus in the elderly population: the KORA S4/F4 cohort study. Eur. J. Epidemiol. 25, 393–402 (2010).
Kermah, D., Shaheen, M., Pan, D. & Friedman, T. C. Multivariate data analysis from the National Health and Nutrition Examination Survey (NHANES) 2001–2006 shows that second-hand smoke is associated with both obesity and diabetes mellitus. Endocrine Society [online], (2012).
Facchini, F. S., Hollenbeck, C. B., Jeppesen, J., Chen, Y. D. & Reaven, G. M. Insulin resistance and cigarette smoking. Lancet 339, 1128–1130 (1992).
Assali, A. R., Beigel, Y., Schreibman, R., Shafer, Z. & Fainaru, M. Weight gain and insulin resistance during nicotine replacement therapy. Clin. Cardiol. 22, 357–360 (1999).
Attvall, S., Fowelin, J., Lager, I., Von Schenck, H. & Smith, U. Smoking induces insulin resistance — a potential link with the insulin resistance syndrome. J. Intern. Med. 233, 327–332 (1993).
Hautanen, A. & Adlercreutz, H. Hyperinsulinaemia, dyslipidaemia and exaggerated adrenal androgen response to adrenocorticotropin in male smokers. Diabetologia 36, 1275–1281 (1993).
Janzon, L., Berntorp, K., Hanson, M., Lindell, S. E. & Trell, E. Glucose tolerance and smoking: a population study of oral and intravenous glucose tolerance tests in middle-aged men. Diabetologia 25, 86–88 (1983).
Kong, C. et al. Smoking is associated with increased hepatic lipase activity, insulin resistance, dyslipidaemia and early atherosclerosis in type 2 diabetes. Atherosclerosis 156, 373–378 (2001).
Ronnemaa, T., Ronnemaa, E. M., Puukka, P., Pyorala, K. & Laakso, M. Smoking is independently associated with high plasma insulin levels in nondiabetic men. Diabetes Care 19, 1229–1232 (1996).
Targher, G. et al. Cigarette smoking and insulin resistance in patients with noninsulin-dependent diabetes mellitus. J. Clin. Endocrinol. Metab. 82, 3619–3624 (1997).
Laws, A. & Reaven, G. M. Evidence for an independent relationship between insulin resistance and fasting plasma HDL-cholesterol, triglyceride and insulin concentrations. J. Intern. Med. 231, 25–30 (1992).
Steinberg, H. O. et al. Obesity/insulin resistance is associated with endothelial dysfunction. Implications for the syndrome of insulin resistance. J. Clin. Invest. 97, 2601–2610 (1996).
Sinha-Hikim, I. et al. Nicotine in combination with a high-fat diet causes intramyocellular mitochondrial abnormalities in male mice. Endocrinology 155, 865–872 (2014).
Farrell, G. C., Teoh, N. C. & McCuskey, R. S. Hepatic microcirculation in fatty liver disease. Anat. Rec. (Hoboken) 291, 684–692 (2008).
Postic, C. & Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: lessons from genetically engineered mice. J. Clin. Invest. 118, 829–838 (2008).
Trauner, M., Arrese, M. & Wagner, M. Fatty liver and lipotoxicity. Biochim. Biophys. Acta 1801, 299–310 (2010).
Liu, R. H., Mizuta, M. & Matsukura, S. Long-term oral nicotine administration reduces insulin resistance in obese rats. Eur. J. Pharmacol. 458, 227–234 (2003).
Nakhate, K. T., Dandekar, M. P., Kokare, D. M. & Subhedar, N. K. Involvement of neuropeptide YY1 receptors in the acute, chronic and withdrawal effects of nicotine on feeding and body weight in rats. Eur. J. Pharmacol. 609, 78–87 (2009).
Seeley, R. J. & Sandoval, D. A. Neuroscience: weight loss through smoking. Nature 475, 176–177 (2011).
Chen, H. et al. Long-term cigarette smoke exposure increases uncoupling protein expression but reduces energy intake. Brain Res. 1228, 81–88 (2008).
Ypsilantis, P. et al. Effects of cigarette smoke exposure and its cessation on body weight, food intake and circulating leptin, and ghrelin levels in the rat. Nicotine Tob. Res. 15, 206–212 (2013).
Audrain-McGovern, J. & Benowitz, N. L. Cigarette smoking, nicotine, and body weight. Clin. Pharmacol. Ther. 90, 164–168 (2011).
Bajaj, M. Nicotine and insulin resistance: when the smoke clears. Diabetes 61, 3078–3080 (2012).
Hankey, C. & Leslie, W. Obesity: is weight gain after smoking cessation an important concern? Nat. Rev. Endocrinol. 8, 630–632 (2012).
Clair, C. et al. Dose-dependent positive association between cigarette smoking, abdominal obesity and body fat: cross-sectional data from a population-based survey. BMC Public Health 11, 23 (2011).
Tsuji, T. et al. Macrophage elastase suppresses white adipose tissue expansion with cigarette smoking. Am. J. Respir. Cell Mol. Biol. 51, 822–829 (2014).
McGrath-Morrow, S. A. et al. The effects of electronic cigarette emissions on systemic cotinine levels, weight and postnatal lung growth in neonatal mice. PLoS ONE 10, e0118344 (2015).
Martinez de Morentin, P. B. et al. Nicotine induces negative energy balance through hypothalamic AMP-activated protein kinase. Diabetes 61, 807–817 (2012).
Schoenberg, N. E., Huang, B., Seshadri, S. & Tucker, T. C. Trends in cigarette smoking and obesity in Appalachian Kentucky. South. Med. J. 108, 170–177 (2015).
Jamal, A. et al. Current cigarette smoking among adults — United States, 2005–2013. MMWR Morb. Mortal. Wkly Rep. 63, 1108–1112 (2014).
Centers for Disease Control and Prevention. Quitting smoking [online], (2015).
Bilano, V. et al. Global trends and projections for tobacco use, 1990–2025: an analysis of smoking indicators from the WHO Comprehensive Information Systems for Tobacco Control. Lancet 385, 966–976 (2015).
US Department of Health and Human Services. Treating tobacco use and dependence: 2008 update. National Center for Biotechnology Information [online], (2008).
Fiore, M. C., Fleming, M. F. & Burns, M. E. Tobacco and alcohol abuse: clinical opportunities for effective intervention. Proc. Assoc. Am. Physicians 111, 131–140 (1999).
Casella, G., Caponnetto, P. & Polosa, R. Therapeutic advances in the treatment of nicotine addiction: present and future. Ther. Adv. Chronic Dis. 1, 95–106 (2010).
Siahpush, M. et al. It is better to be a fat ex-smoker than a thin smoker: findings from the 1997–2004 National Health Interview Survey-National Death Index linkage study. Tob. Control 23, 395–402 (2014).
Tian, J., Venn, A., Otahal, P. & Gall, S. The association between quitting smoking and weight gain: a systemic review and meta-analysis of prospective cohort studies. Obes. Rev. 16, 883–901 (2015).
Klesges, R. C., Meyers, A. W., Klesges, L. M. & La Vasque, M. E. Smoking, body weight, and their effects on smoking behavior: a comprehensive review of the literature. Psychol. Bull. 106, 204–230 (1989).
Williamson, D. F. et al. Smoking cessation and severity of weight gain in a national cohort. N. Engl. J. Med. 324, 739–745 (1991).
Aubin, H. J., Farley, A., Lycett, D., Lahmek, P. & Aveyard, P. Weight gain in smokers after quitting cigarettes: meta-analysis. BMJ 345, e4439 (2012).
Lichtenstein, E., Zhu, S. H. & Tedeschi, G. J. Smoking cessation quitlines: an underrecognized intervention success story. Am. Psychol. 65, 252–261 (2010).
Levine, M. D., Bush, T., Magnusson, B., Cheng, Y. & Chen, X. Smoking-related weight concerns and obesity: differences among normal weight, overweight, and obese smokers using a telephone tobacco quitline. Nicotine Tob. Res. 15, 1136–1140 (2013).
Bush, T. M. et al. Impact of baseline weight on smoking cessation and weight gain in quitlines. Ann. Behav. Med. 47, 208–217 (2014).
Lycett, D., Munafò, M., Johnstone, E., Murphy, M. & Aveyard, P. Associations between weight change over 8 years and baseline body mass index in a cohort of continuing and quitting smokers. Addiction 106, 188–196 (2011).
Locatelli, I., Collet, T. H., Clair, C., Rodondi, N. & Cornuz, J. The joint influence of gender and amount of smoking on weight gain one year after smoking cessation. Int. J. Environ. Res. Public Health 11, 8443–8455 (2014).
Komiyama, M. et al. Analysis of factors that determine weight gain during smoking cessation therapy. PLoS ONE 8, e72010 (2013).
Prod'hom, S. et al. Predictors of weight change in sedentary smokers receiving a standard smoking cessation intervention. Nicotine Tob. Res. 15, 910–916 (2013).
Hur, Y. N., Hong, G. H., Choi, S. H., Shin, K. H. & Chun, B. G. High fat diet altered the mechanism of energy homeostasis induced by nicotine and withdrawal in C57BL/6 mice. Mol. Cells 30, 219–226 (2010).
Lerman, C. et al. Changes in food reward following smoking cessation: a pharmacogenetic investigation. Psychopharmacology (Berl.) 174, 571–577 (2004).
Volkow, N. D., Wang, G. J., Fowler, J. S. & Telang, F. Overlapping neuronal circuits in addiction and obesity: evidence of systems pathology. Phil. Trans. R. Soc. B 363, 3191–3200 (2008).
Johnson, P. M., Hollander, J. A. & Kenny, P. J. Decreased brain reward function during nicotine withdrawal in C57BL6 mice: evidence from intracranial self-stimulation (ICSS) studies. Pharmacol. Biochem. Behav. 90, 409–415 (2008).
White, M. A., Masheb, R. M. & Grilo, C. M. Self-reported weight gain following smoking cessation: a function of binge eating behavior. Int. J. Eat. Disord. 43, 572–575 (2010).
Brook, J. S., Zhang, C., Brook, D. W. & Finch, S. J. Voluntary smoking bans at home and in the car and smoking cessation, obesity, and self-control. Psychol. Rep. 114, 20–31 (2014).
Stadler, M. et al. Effects of smoking cessation on β-cell function, insulin sensitivity, body weight, and appetite. Eur. J. Endocrinol. 170, 219–227 (2014).
Centers for Disease Control and Prevention. Vital signs: nonsmokers' exposure to secondhand smoke — United States, 1999–2008. MMWR Morb. Mortal. Wkly. Rep. 59, 1141–1146 (2010).
Biedermann, L. et al. Smoking cessation induces profound changes in the composition of the intestinal microbiota in humans. PLoS ONE 8, e59260 (2013).
Biedermann, L. et al. Smoking cessation alters intestinal microbiota: insights from quantitative investigations on human fecal samples using FISH. Inflamm. Bowel Dis. 20, 1496–1501 (2014).
Farley, A. C., Hajek, P., Lycett, D. & Aveyard, P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 1, CD006219 (2012).
Love, S. J. et al. Offer of a weight management program to overweight and obese weight-concerned smokers improves tobacco dependence treatment outcomes. Am. J. Addict. 20, 1–8 (2011).
Perkins, K. A. et al. Cognitive-behavioral therapy to reduce weight concerns improves smoking cessation outcome in weight-concerned women. J. Consult. Clin. Psychol. 69, 604–613 (2001).
Meyers, A. W. et al. Are weight concerns predictive of smoking cessation? A prospective analysis. J. Consult. Clin. Psychol. 65, 448–452 (1997).
Parsons, A. C., Shraim, M., Inglis, J., Aveyard, P. & Hajek, P. Interventions for preventing weight gain after smoking cessation. Cochrane Database Syst. Rev. 1, CD006219 (2012).
Levine, M. D. et al. Bupropion and cognitive behavioral therapy for weight-concerned women smokers. Arch. Intern. Med. 170, 543–550 (2010).
Schnoll, R. A., Wileyto, E. P. & Lerman, C. Extended duration therapy with transdermal nicotine may attenuate weight gain following smoking cessation. Addict. Behav. 37, 565–568 (2012).
Taniguchi, C. et al. Varenicline is more effective in attenuating weight gain than nicotine patch 12 months after the end of smoking cessation therapy: an observational study in Japan. Nicotine Tob. Res. 16, 1026–1029 (2014).
Heffner, J. L., Lewis, D. F. & Winhusen, T. M. Osmotic release oral system methylphenidate prevents weight gain during a smoking-cessation attempt in adults with ADHD. Nicotine Tob. Res. 15, 583–587 (2013).
Vergnaud, A. C. et al. Fruit and vegetable consumption and prospective weight change in participants of the European Prospective Investigation into Cancer and Nutrition-Physical Activity, Nutrition, Alcohol, Cessation of Smoking, Eating Out of Home, and Obesity study. Am. J. Clin. Nutr. 95, 184–193 (2012).
Leslie, W. S. et al. Changes in body weight and food choice in those attempting smoking cessation: a cluster randomised controlled trial. BMC Public Health 12, 389 (2012).
Deputy, N. P., Sharma, A. J., Kim, S. Y. & Hinkle, S. N. Prevalence and characteristics associated with gestational weight gain adequacy. Obstet. Gynecol. 125, 773–781 (2015).
Suzuki, K. et al. Effect of maternal smoking cessation before and during early pregnancy on fetal and childhood growth. J. Epidemiol. 24, 60–66 (2014).
Wang, L., Mamudu, H. M. & Wu, T. The impact of maternal prenatal smoking on the development of childhood overweight in school-aged children. Pediatr. Obes. 8, 178–188 (2013).
Hawkins, S. S., Baum, C. F., Oken, E. & Gillman, M. W. Associations of tobacco control policies with birth outcomes. JAMA Pediatr. 168, e142365 (2014).
Lee, K. et al. Associations of smoking and smoking cessation with CT-measured visceral obesity in 4,656 Korean men. Prev. Med. 55, 183–187 (2012).
Matsushita, Y. et al. Associations of smoking cessation with visceral fat area and prevalence of metabolic syndrome in men: the Hitachi health study. Obesity (Silver Spring) 19, 647–651 (2011).
Huang, P. L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2, 231–237 (2009).
Lihn, A. S., Pedersen, S. B. & Richelsen, B. Adiponectin: action, regulation and association to insulin sensitivity. Obes. Rev. 6, 13–21 (2005).
Maeda, K. et al. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (adipose most abundant gene transcript 1). 1996. Biochem. Biophys. Res. Commun. 425, 556–559 (2012).
Shapiro, L. & Scherer, P. E. The crystal structure of a complement-1q family protein suggests an evolutionary link to tumor necrosis factor. Curr. Biol. 8, 335–338 (1998).
Díez, J. J. & Iglesias, P. The role of the novel adipocyte-derived hormone adiponectin in human disease. Eur. J. Endocrinol. 148, 293–300 (2003).
Chen, J. et al. Secretion of adiponectin by human placenta: differential modulation of adiponectin and its receptors by cytokines. Diabetologia 49, 1292–1302 (2006).
Kaur, J. A comprehensive review on metabolic syndrome. Cardiol. Res. Pract. 2014, 943162 (2014).
Inoue, K. et al. Early effects of smoking cessation and weight gain on plasma adiponectin levels and insulin resistance. Intern. Med. 50, 707–712 (2011).
Matthews, D. R. et al. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28, 412–419 (1985).
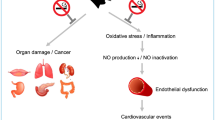
Benowitz, N. L. Cigarette smoking and cardiovascular disease: pathophysiology and implications for treatment. Prog. Cardiovasc. Dis. 46, 91–111 (2003).
Nakanishi, K., Nishida, M., Ohama, T., Moriyama, T. & Yamauchi-Takihara, K. Smoking associates with visceral fat accumulation especially in women. Circ. J. 78, 1259–1263 (2014).
Yun, J. E., Kimm, H., Choi, Y. J., Jee, S. H. & Huh, K. B. Smoking is associated with abdominal obesity, not overall obesity, in men with type 2 diabetes. J. Prev. Med. Public Health 45, 316–322 (2012).
Kirilly, E., Gonda, X. & Bagdy, G. CB1 receptor antagonists: new discoveries leading to new perspectives. Acta Physiol. (Oxf.) 205, 41–60 (2012).
Gamaleddin, I. H. et al. Role of the endogenous cannabinoid system in nicotine addiction: novel insights. Front. Psychiatry 6, 41 (2015).
Silvestri, C. & Di Marzo, V. Second generation CB1 receptor blockers and other inhibitors of peripheral endocannabinoid overactivity and the rationale of their use against metabolic disorders. Expert Opin. Investig. Drugs 21, 1309–1322 (2012).
Garwood, C. L. & Potts, L. A. Emerging pharmacotherapies for smoking cessation. Am. J. Health Syst. Pharm. 64, 1693–1698 (2007).
Bruin, J. E., Gerstein, H. C., Morrison, K. M. & Holloway, A. C. Increased pancreatic β-cell apoptosis following fetal and neonatal exposure to nicotine is mediated via the mitochondria. Toxicol. Sci. 103, 362–370 (2008).
Yoshikawa, H., Hellstrom-Lindahl, E. & Grill, V. Evidence for functional nicotinic receptors on pancreatic β cells. Metabolism 54, 247–254 (2005).
Woynillowicz, A. K., Raha, S., Nicholson, C. J. & Holloway, A. C. The effect of smoking cessation pharmacotherapies on pancreatic β-cell function. Toxicol. Appl. Pharmacol. 265, 122–127 (2012).
Tjalve, H. & Popov, D. Effect of nicotine and nicotine metabolites on insulin secretion from rabbit pancreas pieces. Endocrinology 92, 1343–1348 (1973).
Wu, Y. et al. Activation of AMPKα2 in adipocytes is essential for nicotine-induced insulin resistance in vivo. Nat. Med. 21, 373–382 (2015).
Hsia, S., DesNoyers, M., Lee, M. L., Goldstein, C. & Friedman, T. C. Metabolic effects of smokers undergoing smoking cessation. Endocrine Society [online], (2015).
Bergman, B. C. et al. Novel and reversible mechanisms of smoking-induced insulin resistance in humans. Diabetes 61, 3156–3166 (2012).
Voulgari, C., Katsilambros, N. & Tentolouris, N. Smoking cessation predicts amelioration of microalbuminuria in newly diagnosed type 2 diabetes mellitus: a 1-year prospective study. Metabolism 60, 1456–1464 (2011).
Athyros, V. G., Katsiki, N., Doumas, M., Karagiannis, A. & Mikhailidis, D. P. Effect of tobacco smoking and smoking cessation on plasma lipoproteins and associated major cardiovascular risk factors: a narrative review. Curr. Med. Res. Opin. 29, 1263–1274 (2013).
Legislative Analyst's Office. 2011 Cal Facts. California's economy and budget in perspective. [online], (2011).
Tweed, J. O., Hsia, S. H., Lutfy, K. & Friedman, T. C. The endocrine effects of nicotine and cigarette smoke. Trends Endocrinol. Metab. 23, 334–342 (2012).
Acknowledgements
Salary support for T.C.F. was provided by the Diversity-promoting Institutions Drug Abuse Research Development Program (grant R24DA017298) and the National Institute on Minority Health and Health Disparities (grant U54MD007598). The authors acknowledge the professional development core of the Charles R. Drew University Accelerating Excellence in Translational Science (AXIS) (grant U54MD007598) for help with editing the manuscript.
Author information
Authors and Affiliations
Contributions
K.K.H., M.Z. and T.C.F. researched data for the article. K.K.H. and T.C.F. provided substantial contributions to discussions of the content. K.K.H., M.Z. and T.C.F. wrote the article and reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Harris, K., Zopey, M. & Friedman, T. Metabolic effects of smoking cessation. Nat Rev Endocrinol 12, 299–308 (2016). https://doi.org/10.1038/nrendo.2016.32
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2016.32
This article is cited by
-
Utilization of the microbiome in personalized medicine
Nature Reviews Microbiology (2024)
-
When smoke meets gut: deciphering the interactions between tobacco smoking and gut microbiota in disease development
Science China Life Sciences (2024)
-
Glucagon-like peptide-1 analogues: a new way to quit smoking? (SKIP)—a structured summary of a study protocol for a randomized controlled study
Trials (2023)
-
Association of post-smoking cessation changes in fasting serum glucose with changes in predicted fatty liver score
Scientific Reports (2023)
-
Contribution of environmental, genetic and epigenetic factors to obesity-related metabolic syndrome
The Nucleus (2023)