Key Points
-
Hypothalamic metabolic sensing requires an interactive network of neurons, glial cells and vasculature to enable appropriate integration of complex metabolic feedback signals
-
Exposure to hypercaloric environments induces inflammatory-like responses not only in peripheral tissues but also in the central nervous system, especially in the hypothalamus
-
The term 'hypothalamic inflammation' is being used more and more frequently to describe a complex of hypothalamic processes that occurs in response to hypercaloric diets
-
The phenomenon of hypothalamic inflammation in obesity is more similar to an innate immune reaction than to conventionally defined inflammation
-
To address the hypothalamic innate immune reaction therapeutically, cell-specific strategies should be developed that enable prevention of the adverse effects of systemic treatment approaches
Abstract
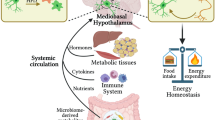
Findings from rodent and human studies show that the presence of inflammatory factors is positively correlated with obesity and the metabolic syndrome. Obesity-associated inflammatory responses take place not only in the periphery but also in the brain. The hypothalamus contains a range of resident glial cells including microglia, macrophages and astrocytes, which are embedded in highly heterogenic groups of neurons that control metabolic homeostasis. This complex neural–glia network can receive information directly from blood-borne factors, positioning it as a metabolic sensor. Following hypercaloric challenge, mediobasal hypothalamic microglia and astrocytes enter a reactive state, which persists during diet-induced obesity. In established mouse models of diet-induced obesity, the hypothalamic vasculature displays angiogenic alterations. Moreover, proopiomelanocortin neurons, which regulate food intake and energy expenditure, are impaired in the arcuate nucleus, where there is an increase in local inflammatory signals. The sum total of these events is a hypothalamic innate immune reactivity, which includes temporal and spatial changes to each cell population. Although the exact role of each participant of the neural–glial–vascular network is still under exploration, therapeutic targets for treating obesity should probably be linked to individual cell types and their specific signalling pathways to address each dysfunction with cell-selective compounds.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zhang, Y. et al. Positional cloning of the mouse obese gene and its human homologue. Nature 372, 425–432 (1994).
Williams, K. W. & Elmquist, J. K. From neuroanatomy to behavior: central integration of peripheral signals regulating feeding behavior. Nat. Neurosci. 15, 1350–1355 (2012).
Begg, D. P. & Woods, S. C. The endocrinology of food intake. Nat. Rev. Endocrinol. 9, 584–597 (2013).
Furuhashi, M. et al. Adipocyte/macrophage fatty acid-binding proteins contribute to metabolic deterioration through actions in both macrophages and adipocytes in mice. J. Clin. Invest. 118, 2640–2650 (2008).
Mathis, D. Immunological goings-on in visceral adipose tissue. Cell Metab. 17, 851–859 (2013).
Zhang, X. et al. Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135, 61–73 (2008).
De Souza, C. T. et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology 146, 4192–4199 (2005).
Yi, C. X., Zeltser, L. & Tschöp, M. H. Metabolic Syndrome ePoster—Brain & Neuron. Nat. Med. [online], (2011).
Yi, C. X. & Tschöp, M. H. Brain-gut-adipose-tissue communication pathways at a glance. Dis. Model. Mech. 5, 583–587 (2012).
Yi, C. X., Scherer, T. & Tschöp, M. H. Cajal revisited: does the VMH make us fat? Nat. Neurosci. 14, 806–808 (2011).
Kim, K. W. et al. CNS-specific ablation of steroidogenic factor 1 results in impaired female reproductive function. Mol. Endocrinol. 24, 1240–1250 (2010).
Xu, B. et al. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 6, 736–742 (2003).
Obici, S., Zhang, B. B., Karkanias, G. & Rossetti, L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat. Med. 8, 1376–1382 (2002).
Bruinstroop, E. et al. The autonomic nervous system regulates postprandial hepatic lipid metabolism. Am. J. Physiol. Endocrinol. Metab. 304, E1089–E1096 (2013).
Lam, T. K. et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat. Med. 11, 320–327 (2005).
Cottrell, G. T. & Ferguson, A. V. Sensory circumventricular organs: central roles in integrated autonomic regulation. Regul. Pept. 117, 11–23 (2004).
Milanski, M. et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J. Neurosci. 29, 359–370 (2009).
Thaler, J. P. et al. Obesity is associated with hypothalamic injury in rodents and humans. J. Clin. Invest. 122, 153–162 (2012).
Yi, C. X. et al. High calorie diet triggers hypothalamic angiopathy. Mol. Metab. 1, 95–100 (2012).
Moraes, J. C. et al. High-fat diet induces apoptosis of hypothalamic neurons. PLoS ONE 4, e5045 (2009).
Li, J., Tang, Y. & Cai, D. IKKβ/NF-κB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat. Cell Biol. 14, 999–1012 (2012).
Heneka, M. T., Kummer, M. P. & Latz, E. Innate immune activation in neurodegenerative disease. Nat. Rev. Immunol. 14, 463–477 (2014).
Heppner, F., Ransohoff, R. & Becher, B. Immune attack! The role of inflammation in Alzheimer's disease. Nat. Rev. Neurosci. (in press).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013).
Alliot, F., Godin, I. & Pessac, B. Microglia derive from progenitors, originating from the yolk sac, and which proliferate in the brain. Brain Res. Dev. Brain Res. 117, 145–152 (1999).
Perry, V. H., Hume, D. A. & Gordon, S. Immunohistochemical localization of macrophages and microglia in the adult and developing mouse brain. Neuroscience 15, 313–326 (1985).
Massberg, S. et al. Immunosurveillance by hematopoietic progenitor cells trafficking through blood, lymph, and peripheral tissues. Cell 131, 994–1008 (2007).
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007).
Varvel, N. H. et al. Microglial repopulation model reveals a robust homeostatic process for replacing CNS myeloid cells. Proc. Natl Acad. Sci. USA 109, 18150–18155 (2012).
Li, Y., Du, X. F., Liu, C. S., Wen, Z. L. & Du, J. L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell 23, 1189–1202 (2012).
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014).
Kettenmann, H., Hanisch, U. K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
Daikoku, S., Kotsu, T. & Hashimoto, M. Electron microscopic observations on the development of the median eminence in perinatal rats. Z. Anat. Entwicklungsgesch. 134, 311–327 (1971).
Bitsch, P. & Schiebler, T. H. Postnatal development of the median eminence in the rat [German]. Z. Mikrosk. Anat. Forsch. 93, 1–20 (1979).
Pow, D. V., Perry, V. H., Morris, J. F. & Gordon, S. Microglia in the neurohypophysis associate with and endocytose terminal portions of neurosecretory neurons. Neuroscience 33, 567–578 (1989).
Jung, S. et al. Analysis of fractalkine receptor CX3CR1 function by targeted deletion and green fluorescent protein reporter gene insertion. Mol. Cell. Biol. 20, 4106–4114 (2000).
Hawkes, C. A. & McLaurin, J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 106, 1261–1266 (2009).
Grossmann, R. et al. Juxtavascular microglia migrate along brain microvessels following activation during early postnatal development. Glia 37, 229–240 (2002).
Davalos, D. et al. Fibrinogen-induced perivascular microglial clustering is required for the development of axonal damage in neuroinflammation. Nat. Commun. 3, 1227 (2012).
Neumann, H., Kotter, M. R. & Franklin, R. J. Debris clearance by microglia: an essential link between degeneration and regeneration. Brain 132, 288–295 (2009).
Douglas, S. D. & Musson, R. A. Phagocytic defects--monocytes/macrophages. Clin. Immunol. Immunopathol. 40, 62–68 (1986).
Vedeler, C. et al. Fc receptor for IgG (FcR) on rat microglia. J. Neuroimmunol. 49, 19–24 (1994).
Quan, Y., Moller, T. & Weinstein, J. R. Regulation of Fcγ receptors and immunoglobulin G-mediated phagocytosis in mouse microglia. Neurosci. Lett. 464, 29–33 (2009).
Webster, S. D., Park, M., Fonseca, M. I. & Tenner, A. J. Structural and functional evidence for microglial expression of C1qRP, the C1q receptor that enhances phagocytosis. J. Leukoc. Biol. 67, 109–116 (2000).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
Citron, M. Alzheimer's disease: strategies for disease modification. Nat. Rev. Drug Discov. 9, 387–398 (2010).
Fishman, P. S. & Savitt, J. M. Selective localization by neuroglia of immunoglobulin G in normal mice. J. Neuropathol. Exp. Neurol. 48, 212–220 (1989).
Yi, C. X., Tschöp, M. H., Woods, S. C. & Hofmann, S. M. High-fat-diet exposure induces IgG accumulation in hypothalamic microglia. Dis. Model. Mech. 5, 686–690 (2012).
Ferreira, R. et al. Neuropeptide Y inhibits interleukin-1β-induced phagocytosis by microglial cells. J. Neuroinflammation 8, 169 (2011).
Krabbe, G. et al. Functional impairment of microglia coincides with β-amyloid deposition in mice with Alzheimer-like pathology. PLoS ONE 8, e60921 (2013).
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009).
Paolicelli, R. C. et al. Synaptic pruning by microglia is necessary for normal brain development. Science 333, 1456–1458 (2011).
Zhan, Y. et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Bouret, S. G., Draper, S. J. & Simerly, R. B. Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J. Neurosci. 24, 2797–2805 (2004).
Loffreda, S. et al. Leptin regulates proinflammatory immune responses. FASEB J. 12, 57–65 (1998).
Schafer, D. P. et al. Microglia sculpt postnatal neural circuits in an activity and complement-dependent manner. Neuron 74, 691–705 (2012).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005).
Schaeffer, M. et al. Rapid sensing of circulating ghrelin by hypothalamic appetite-modifying neurons. Proc. Natl Acad. Sci. USA 110, 1512–1517 (2013).
Gao, Y. et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia 62, 17–25 (2014).
Lafrance, V., Inoue, W., Kan, B. & Luheshi, G. N. Leptin modulates cell morphology and cytokine release in microglia. Brain Behav. Immun. 24, 358–365 (2010).
Kleinridders, A. et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 10, 249–259 (2009).
Choi, S. J., Kim, F., Schwartz, M. W. & Wisse, B. E. Cultured hypothalamic neurons are resistant to inflammation and insulin resistance induced by saturated fatty acids. Am. J. Physiol. Endocrinol. Metab. 298, E1122–E1130 (2010).
Douard, V. & Ferraris, R. P. Regulation of the fructose transporter GLUT5 in health and disease. Am. J. Physiol. Endocrinol. Metab. 295, E227–E237 (2008).
Maher, F., Vannucci, S. J. & Simpson, I. A. Glucose transporter proteins in brain. FASEB J. 8, 1003–1011 (1994).
Vannucci, S. J., Maher, F. & Simpson, I. A. Glucose transporter proteins in brain: delivery of glucose to neurons and glia. Glia 21, 2–21 (1997).
Vom Berg, J. et al. Inhibition of IL-12/IL-23 signaling reduces Alzheimer's disease-like pathology and cognitive decline. Nat. Med. 18, 1812–1819 (2012).
Zhang, B. et al. Integrated systems approach identifies genetic nodes and networks in late-onset Alzheimer's disease. Cell 153, 707–720 (2013).
Streit, W. J., Braak, H., Xue, Q. S. & Bechmann, I. Dystrophic (senescent) rather than activated microglial cells are associated with tau pathology and likely precede neurodegeneration in Alzheimer's disease. Acta Neuropathol. 118, 475–485 (2009).
Parkhurst, C. N. et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell 155, 1596–1609 (2013).
De Filippo, C. et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl Acad. Sci. USA 107, 14691–14696 (2010).
Zhao, L. The gut microbiota and obesity: from correlation to causality. Nat. Rev. Microbiol. 11, 639–647 (2013).
Inoue, K. Microglial activation by purines and pyrimidines. Glia 40, 156–163 (2002).
James, G. & Butt, A. M. P2Y and P2X purinoceptor mediated Ca2+ signalling in glial cell pathology in the central nervous system. Eur. J. Pharmacol. 447, 247–260 (2002).
Samuels, S. E., Lipitz, J. B., Wang, J., Dahl, G. & Muller, K. J. Arachidonic acid closes innexin/pannexin channels and thereby inhibits microglia cell movement to a nerve injury. Dev. Neurobiol. 73, 621–631 (2013).
Bermudez-Silva, F. J., Cardinal, P. & Cota, D. The role of the endocannabinoid system in the neuroendocrine regulation of energy balance. J. Psychopharmacol. 26, 114–124 (2012).
Cardona, A. E. et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 (2006).
Morari, J. et al. Fractalkine (CX3CL1) is involved in the early activation of hypothalamic inflammation in experimental obesity. Diabetes 63, 3770–3784 (2014).
Ruchaya, P. J., Antunes, V. R., Paton, J. F., Murphy, D. & Yao, S. T. The cardiovascular actions of fractalkine/CX3CL1 in the hypothalamic paraventricular nucleus are attenuated in rats with heart failure. Exp. Physiol. 99, 111–122 (2014).
Buckman, L. B. et al. Obesity induced by a high-fat diet is associated with increased immune cell entry into the central nervous system. Brain Behav. Immun. 35, 33–42 (2014).
Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease. J. Neurosci. 31, 11159–11171 (2011).
Kierdorf, K., Katzmarski, N., Haas, C. A. & Prinz, M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS ONE 8, e58544 (2013).
Gordon, S. Alternative activation of macrophages. Nat. Rev. Immunol. 3, 23–35 (2003).
Murray, P. J. & Wynn, T. A. Protective and pathogenic functions of macrophage subsets. Nat. Rev. Immunol. 11, 723–737 (2011).
Michelucci, A., Heurtaux, T., Grandbarbe, L., Morga, E. & Heuschling, P. Characterization of the microglial phenotype under specific pro-inflammatory and anti-inflammatory conditions: effects of oligomeric and fibrillar amyloid-β. J. Neuroimmunol. 210, 3–12 (2009).
Ponomarev, E. D., Maresz, K., Tan, Y. & Dittel, B. N. CNS-derived interleukin-4 is essential for the regulation of autoimmune inflammation and induces a state of alternative activation in microglial cells. J. Neurosci. 27, 10714–10721 (2007).
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Town, T., Nikolic, V. & Tan, J. The microglial “activation” continuum: from innate to adaptive responses. J. Neuroinflammation 2, 24 (2005).
Murray, P. J. et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41, 14–20 (2014).
Wieghofer, P., Knobeloch, K. P. & Prinz, M. Genetic targeting of microglia. Glia 63, 1–22 (2014).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Heppner, F. L. et al. Experimental autoimmune encephalomyelitis repressed by microglial paralysis. Nat. Med. 11, 146–152 (2005).
Grathwohl, S. A. et al. Formation and maintenance of Alzheimer's disease beta-amyloid plaques in the absence of microglia. Nat. Neurosci. 12, 1361–1363 (2009).
Yi, C. X., Habegger, K. M., Chowen, J. A., Stern, J. & Tschöp, M. H. A role for astrocytes in the central control of metabolism. Neuroendocrinology 93, 143–149 (2011).
Broer, S. et al. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. Expression of two different monocarboxylate transporters in astroglial cells and neurons. J. Biol. Chem. 272, 30096–30102 (1997).
Le Foll, C., Dunn-Meynell, A. A., Miziorko, H. M. & Levin, B. E. Regulation of hypothalamic neuronal sensing and food intake by ketone bodies and fatty acids. Diabetes 63, 1259–1269 (2014).
Dixit, V. D. et al. Ghrelin and the growth hormone secretagogue receptor constitute a novel autocrine pathway in astrocytoma motility. J. Biol. Chem. 281, 16681–16690 (2006).
Hsuchou, H. et al. Obesity induces functional astrocytic leptin receptors in hypothalamus. Brain 132, 889–902 (2009).
Heni, M. et al. Insulin promotes glycogen storage and cell proliferation in primary human astrocytes. PLoS ONE 6, e21594 (2011).
Kim, J. G. et al. Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding. Nat. Neurosci. 17, 908–910 (2014).
Diano, S., Leonard, J. L., Meli, R., Esposito, E. & Schiavo, L. Hypothalamic type II iodothyronine deiodinase: a light and electron microscopic study. Brain Res. 976, 130–134 (2003).
Herwig, A., Ross, A. W., Nilaweera, K. N., Morgan, P. J. & Barrett, P. Hypothalamic thyroid hormone in energy balance regulation. Obes. Facts 1, 71–79 (2008).
Courtin, F. et al. Thyroid hormone deiodinases in the central and peripheral nervous system. Thyroid 15, 931–942 (2005).
Kamphuis, W. et al. GFAP isoforms in adult mouse brain with a focus on neurogenic astrocytes and reactive astrogliosis in mouse models of Alzheimer disease. PLoS ONE 7, e42823 (2012).
Horvath, T. L. et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc. Natl Acad. Sci. USA 107, 14875–14880 (2010).
Frederich, R. C. et al. Leptin levels reflect body lipid content in mice: evidence for diet-induced resistance to leptin action. Nat. Med. 1, 1311–1314 (1995).
Campbell, I. L. et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc. Natl Acad. Sci. USA 90, 10061–10065 (1993).
Haugh, R. M. & Markesbery, W. R. Hypothalamic astrocytoma. Syndrome of hyperphagia, obesity, and disturbances of behavior and endocrine and autonomic function. Arch. Neurol. 40, 560–563 (1983).
Emsley, J. G. & Macklis, J. D. Astroglial heterogeneity closely reflects the neuronal-defined anatomy of the adult murine CNS. Neuron Glia Biol. 2, 175–186 (2006).
Langlet, F. et al. Tanycytic VEGF-A boosts blood-hypothalamus barrier plasticity and access of metabolic signals to the arcuate nucleus in response to fasting. Cell Metab. 17, 607–617 (2013).
Milanski, M. et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes 61, 1455–1462 (2012).
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Ventre, J. et al. Targeted disruption of the tumor necrosis factor-alpha gene: metabolic consequences in obese and nonobese mice. Diabetes 46, 1526–1531 (1997).
Pamir, N., McMillen, T. S., Kaiyala, K. J., Schwartz, M. W. & LeBoeuf, R. C. Receptors for tumor necrosis factor-α play a protective role against obesity and alter adipose tissue macrophage status. Endocrinology 150, 4124–4134 (2009).
Aggarwal, B. B. Signalling pathways of the TNF superfamily: a double-edged sword. Nat. Rev. Immunol. 3, 745–756 (2003).
Baker, R. G., Hayden, M. S. & Ghosh, S. NF-κB, inflammation, and metabolic disease. Cell Metab. 13, 11–22 (2011).
Perkins, N. D. Integrating cell-signalling pathways with NF-κB and IKK function. Nat. Rev. Mol. Cell Biol. 8, 49–62 (2007).
Meng, Q. & Cai, D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IκB kinase β (IKKβ)/NF-κB pathway. J. Biol. Chem. 286, 32324–32332 (2011).
Purkayastha, S., Zhang, G. & Cai, D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-β and NF-κB. Nat. Med. 17, 883–887 (2011).
Koulich, E., Nguyen, T., Johnson, K., Giardina, C. & D'Mello, S. NF-κB is involved in the survival of cerebellar granule neurons: association of Iκβ phosphorylation with cell survival. J. Neurochem. 76, 1188–1198 (2001).
Culmsee, C. et al. Reciprocal inhibition of p53 and nuclear factor-κB transcriptional activities determines cell survival or death in neurons. J. Neurosci. 23, 8586–8595 (2003).
Kuno, R. et al. Autocrine activation of microglia by tumor necrosis factor-α. J. Neuroimmunol. 162, 89–96 (2005).
Listwak, S. J., Rathore, P. & Herkenham, M. Minimal NF-κB activity in neurons. Neuroscience 250, 282–299 (2013).
Tantiwong, P. et al. NF-κB activity in muscle from obese and type 2 diabetic subjects under basal and exercise-stimulated conditions. Am. J. Physiol. Endocrinol. Metab. 299, E794–E801 (2010).
Dietrich, M. O. & Horvath, T. L. Hypothalamic control of energy balance: insights into the role of synaptic plasticity. Trends Neurosci. 36, 65–73 (2013).
Zeltser, L. M., Seeley, R. J. & Tschöp, M. H. Synaptic plasticity in neuronal circuits regulating energy balance. Nat. Neurosci. 15, 1336–1342 (2012).
Mattson, M. P., Gleichmann, M. & Cheng, A. Mitochondria in neuroplasticity and neurological disorders. Neuron 60, 748–766 (2008).
Chang, D. T., Honick, A. S. & Reynolds, I. J. Mitochondrial trafficking to synapses in cultured primary cortical neurons. J. Neurosci. 26, 7035–7045 (2006).
Sheng, Z. H. & Cai, Q. Mitochondrial transport in neurons: impact on synaptic homeostasis and neurodegeneration. Nat. Rev. Neurosci. 13, 77–93 (2012).
de Brito, O. M. & Scorrano, L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature 456, 605–610 (2008).
Vannuvel, K., Renard, P., Raes, M. & Arnould, T. Functional and morphological impact of ER stress on mitochondria. J. Cell. Physiol. 228, 1802–1818 (2013).
Schneeberger, M. et al. Mitofusin 2 in POMC neurons connects ER stress with leptin resistance and energy imbalance. Cell 155, 172–187 (2013).
Diano, S. et al. Peroxisome proliferation-associated control of reactive oxygen species sets melanocortin tone and feeding in diet-induced obesity. Nat. Med. 17, 1121–1127 (2011).
Dietrich, M. O., Liu, Z. W. & Horvath, T. L. Mitochondrial dynamics controlled by mitofusins regulate Agrp neuronal activity and diet-induced obesity. Cell 155, 188–199 (2013).
Cnop, M. et al. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes 54 (Suppl.2), S97–S107 (2005).
Schapira, A. H., Olanow, C. W., Greenamyre, J. T. & Bezard, E. Slowing of neurodegeneration in Parkinson's disease and Huntington's disease: future therapeutic perspectives. Lancet 384, 545–555 (2014).
Alkemade, A. et al. AgRP and NPY expression in the human hypothalamic infundibular nucleus correlate with body mass index, whereas changes in αMSH are related to type 2 diabetes. J. Clin. Endocrinol. Metab. 97, E925–E933 (2012).
Watkins, L. R. & Hutchinson, M. R. A concern on comparing 'apples' and 'oranges' when differences between microglia used in human and rodent studies go far, far beyond simply species: comment on Smith and Dragunow. Trends Neurosci. 37, 189–190 (2014).
Smith, A. M. & Dragunow, M. The human side of microglia. Trends Neurosci. 37, 125–135 (2014).
Finan, B. et al. Targeted estrogen delivery reverses the metabolic syndrome. Nat. Med. 18, 1847–1856 (2012).
Komm, B. S. & Mirkin, S. An overview of current and emerging SERMs. J. Steroid Biochem. Mol. Biol. 143, 207–222 (2014).
van der Goes, A., Hoekstra, K., van den Berg, T. K. & Dijkstra, C. D. Dexamethasone promotes phagocytosis and bacterial killing by human monocytes/macrophages in vitro. J. Leukoc. Biol. 67, 801–807 (2000).
Caro, J. F. & Amatruda, J. M. Glucocorticoid-induced insulin resistance: the importance of postbinding events in the regulation of insulin binding, action, and degradation in freshly isolated and primary cultures of rat hepatocytes. J. Clin. Invest. 69, 866–875 (1982).
Kennedy, B., Elayan, H. & Ziegler, M. G. Glucocorticoid induction of epinephrine synthesizing enzyme in rat skeletal muscle and insulin resistance. J. Clin. Invest. 92, 303–307 (1993).
Yi, C. X. et al. Glucocorticoid signaling in the arcuate nucleus modulates hepatic insulin sensitivity. Diabetes 61, 339–345 (2012).
Acknowledgements
F.L.H. is supported by grants from the Deutsche Forschungsgemeinschaft (SFB TRR 43, NeuroCure Exc 257 and HE 3130/6-1), the Federal Ministry of Education and Research (DLR/BMBF; Kompetenznetz Degenerative Demenzen) and by a collaborative research grant of the Berlin Institute of Health (BIH). I.B. is supported by the Deutsche Forschungsgemeinsschaft (FOR 1336 and SFB 1052), ICEMED and DZD. M.H.T. is supported by the Alexander von Humboldt Foundation, the Deutsches Zentrum für Diabetesforschung (DZD), and the Helmholtz Alliance ICEMED–Imaging and Curing Environmental Metabolic Diseases, through the Initiative and Networking Fund of the Helmholtz Association.
Author information
Authors and Affiliations
Contributions
M.H.T. and C.-X.Y. provided substantial contribution to discussion of the content. S.K. and C.-X.Y. wrote the article. F.L.H., I.B., M.P., M.H.T. and C.-X.Y. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kälin, S., Heppner, F., Bechmann, I. et al. Hypothalamic innate immune reaction in obesity. Nat Rev Endocrinol 11, 339–351 (2015). https://doi.org/10.1038/nrendo.2015.48
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.48
This article is cited by
-
Obesity affects brain cortex gene expression in an APOE genotype and sex dependent manner
International Journal of Obesity (2024)
-
Reduction of oxytocin-containing neurons and enhanced glymphatic activity in the hypothalamic paraventricular nucleus of patients with type 2 diabetes mellitus
Acta Neuropathologica Communications (2023)
-
Metabolism and memory: α-synuclein level in children with obesity and children with type 1 diabetes; relation to glucotoxicity, lipotoxicity and executive functions
International Journal of Obesity (2022)
-
An orally active plant Rubisco-derived peptide increases neuronal leptin responsiveness
Scientific Reports (2022)
-
Metabolic factors in the regulation of hypothalamic innate immune responses in obesity
Experimental & Molecular Medicine (2022)