Key Points
-
The extracellular calcium-sensing receptor (CaSR) expressed in the parathyroid glands and kidneys modulates blood levels of Ca2+; CaSR is also expressed on bone cells, where it regulates skeletal homeostasis
-
In chondrocytes, CaSR contributes to development of the cartilaginous growth plate, whereas in osteoblasts CaSR is required for the proliferation and differentiation of these cells, and for bone matrix production
-
In young animals, activation of CaSR in osteoclasts inhibits bone resorption, which results in enhanced bone anabolism
-
In old animals, although activation of osteoblasts augments bone formation, this activation also increases the expression of receptor activator of nuclear factor κB ligand and bone resorption by osteoclasts
-
The relationship between activation of CaSR in the skeleton and levels of parathyroid hormone can lead to net bone formation in trabecular bone and net bone resorption in cortical bone
-
Although currently available allosteric modulators of CaSR in the parathyroid gland are beneficial in some clinical conditions, future CaSR-based drugs might be developed that have improved effects on bone
Abstract
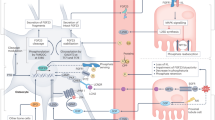
The extracellular calcium-sensing receptor, CaSR, is a member of the G protein-coupled receptor superfamily and has a critical role in modulating Ca2+ homeostasis via its role in the parathyroid glands and kidneys. New evidence suggests that CaSR expression in cartilage and bone also directly regulates skeletal homeostasis. This Review discusses the role of CaSR in chondrocytes, through which CaSR contributes to the development of the cartilaginous growth plate, as well as in osteoblasts and osteoclasts, through which CaSR has effects on skeletal development and bone turnover in young and mature animals. The interaction of skeletal CaSR activation with parathyroid hormone (PTH), which is secreted by the parathyroid gland, can lead to net bone formation in trabecular bone or net bone resorption in cortical bone. Allosteric modulators of CaSR are beneficial in some clinical conditions, with effects that are mediated by the ability of these agents to alter levels of PTH and improve Ca2+ homeostasis. However, further insights into the action of CaSR in bone cells might lead to CaSR-based drugs that maximize not only the effects of the receptor on the parathyroid glands and kidneys but also on bone.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Quinn, S. J. et al. CaSR-mediated interactions between calcium and magnesium homeostasis in mice. Am. J. Physiol. Endocrinol. Metab. 304, E724–E733 (2013).
Aida, K., Koishi, S., Tawata, M. Y. & Onaya, T. Molecular cloning of a putative Ca2+-sensing receptor cDNA from human kidney. Biochem. Biophys. Res. Commun. 214, 524–529 (1995).
Pidasheva, S. et al. Calcium-sensing receptor dimerizes in the endoplasmic reticulum: biochemical and biophysical characterization of novel CASR mutations retained intracellularly. Hum. Mol. Genet. 15, 2200–2209 (2006).
Breitwieser, G. E. The calcium sensing receptor life cycle: trafficking, cell surface expression, and degradation. Best Pract. Res. Clin. Endocrinol. Metab. 27, 303–313 (2013).
Ward, D. T. & Riccardi, D. New concepts in calcium-sensing receptor pharmacology and signaling. Br. J. Pharmacol. 165, 35–48 (2012).
Conigrave, A. D. & Ward, D. T. Calcium-sensing receptor (CaSR): pharmacological properties and signaling pathways. Best Pract. Res. Clin. Endocrinol. Metab. 27, 315–331 (2013).
Brown, E. M. et al. Cloning and characterization of an extracellular Ca2+-sensing receptor from bovine parathyroid. Nature 366, 575–580 (1993).
Brown, E. M. Calcium receptor and regulation of parathyroid hormone secretion. Rev. Endocr. Metab. Disord. 1, 307–315 (2000).
Kantham, L. et al. The calcium-sensing receptor defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am. J. Physiol. Endocrinol. Metab. 297, E915–E923 (2009).
Goltzman, D. Emerging roles for calcium-regulating hormones beyond osteolysis. Trends Endocrinol. Metab. 21, 512–518 (2010).
Meir, T. et al. Parathyroid hormone activates the orphan receptor Nurr1 to induce FGF23 transcription. Kidney Int. 86, 1106–1115 (2014).
Quinn, S. J. et al. Interactions between calcium and phosphorus in the regulation of the production of fibroblast growth factor 23 in vivo. Am. J. Physiol. Endocrinol. Metab. 304, E310–E320 (2013).
Kos, C. H. et al. The calcium-sensing receptor is required for normal calcium homeostasis independent of parathyroid hormone. J. Clin. Invest. 111, 1021–1028 (2003).
Noureldine, S., Genther, D. J., Lopez, M., Agarwal, N. & Tufano, R. P. Early predictors of hypocalcemia after total thyroidectomy: an analysis of 303 patients using a short-stay monitoring protocol. JAMA Otolaryngol. Head Neck Surg. 140, 1006–1013 (2014).
Goltzman, D., Potts, J. T. Jr, Ridgeway, E. C. & Maloof, F. Calcitonin as a tumor marker—use of the radioimmunoassay for calcitonin in the postoperative evaluation of patients with medullary thyroid carcinoma. N. Engl. J. Med. 290, 1035–1039 (1974).
Cole, D. E. et al. A986S polymorphism of the calcium-sensing receptor and circulating calcium concentrations. Lancet 353, 112–115 (1999).
O'Seaghdha, C. M. et al. Common variants in the calcium-sensing receptor are associated with total serum calcium levels. Hum. Mol. Genet. 19, 4296–4303 (2010).
O'Seaghdha, C. M. et al. Meta-analysis of genome-wide association studies identifies six new loci for serum calcium concentrations. PLoS Genet. 9, e1003796 (2013).
Hendy, G. N., Canaff, L. & Cole, D. E. The CASR gene: alternative splicing and transcriptional control, and calcium-sensing receptor (CaSR) protein: structure and ligand binding sites. Best Pract. Res. Clin. Endocrinol. Metab. 27, 285–301 (2013).
Nemeth, E. F. et al. Calcimimetics with potent and selective activity on the parathyroid calcium receptor. Proc. Natl Acad. Sci. USA 95, 4040–4045 (1998).
Nemeth, E. F. Anabolic therapy for osteoporosis: calcilytics. BoneKey 5, 196–208 (2008).
Kumar, S. et al. An orally active calcium-sensing receptor antagonist that transiently increases plasma concentrations of PTH and stimulates bone formation. Bone 46, 534–542 (2010).
Gowen, M. et al. Antagonizing the parathyroid calcium receptor stimulates parathyroid hormone secretion and bone formation in osteopenic rats. J. Clin. Invest. 105, 1595–1604 (2000).
MacGregor, R. R., Jilka, R. L. & Hamilton, J. W. Formation and secretion of fragments of parathormone. Identification of cleavage sites. J. Biol. Chem. 261, 1929–1934 (1986).
Fasciotto, B. H., Denny, J. C., Greeley, G. H. Jr & Cohn. D. V. Processing of chromogranin A in the parathyroid: generation of parastatin-related peptides. Peptides 21, 1389–1401 (2000).
MacGregor, R. R., Hamilton, J. W., Shofstall, R. E. & Cohn, D. V. Isolation and characterization of porcine parathyroid cathepsin B. J. Biol. Chem. 254, 4423–4427 (1979).
Sabbagh, Y., Carpenter, T. O. & Demay, M. B. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc. Natl Acad. Sci. USA 102, 9637–9642 (2005).
Panda, D. K. et al. Inactivation of the 25-hydroxyvitamin D 1α-hydroxylase and vitamin D receptor demonstrates independent and interdependent effects of calcum and vitamin D on skeletal and mineral homeostasis. J. Biol. Chem. 279, 16754–16766 (2004).
Amizuka, N., Warshawsky, H., Henderson, J. E., Goltzman, D. & Karaplis, A. C. Parathyroid hormone-related peptide-depleted mice show abnormal epiphyseal cartilage development and altered endochondral bone formation. J. Cell. Biol. 126, 1611–1623 (1994).
Mamillapalli, R. & Wysolmerski, J. The calcium-sensing receptor couples to Gαs and regulates PTHrP and ACTH secretion in pituitary cells. J. Endocrinol. 204, 287–297 (1994).
Liu, J. et al. The abnormal phenotypes of cartilage and bone in calcium-sensing receptor deficient mice are dependent on the actions of calcium, phosphorus, and PTH. PLoS Genet. 7, e1002294 (2011).
Chang, W. et al. Calcium sensing in cultured chondrogenic RCJ3.1C5.18 cells. Endocrinology 140, 1911–1919 (1999).
Chang, W., Tu, C., Pratt, S., Chen, T. H. & Shoback, D. Extracellular Ca2+-sensing receptors modulate matrix production and mineralization in chondrogenic RCJ3.1C5.18 cells. Endocrinology 143, 1467–1474 (2002).
Rodriguez, L., Cheng, Z., Chen, T. H., Tu, C. & Chang, W. Extracellular calcium and parathyroid hormone-related peptide signaling modulate the pace of growth plate chondrocyte differentiation. Endocrinology 146, 4597–4608 (2005).
Chang, W., Tu, C., Chen, T. H., Bikle, D. & Shoback, D. The extracellular calcium-sensing receptor (CaSR) is a critical modulator of skeletal development. Sci. Signal. 1, ra1 (2008).
Nguyen-Yamamoto, L., Bolivar, I., Srugnell, S. A. & Goltzman, D. Comparison of vitamin D compounds and a calcimimetic in mineral homeostasis. J. Am. Soc. Nephrol. 21, 1713–1723 (2010).
Richard, C. et al. The calcium-sensing receptor and 25-hydroxyvitamin D-1α-hydroxylase interact to modulate skeletal growth and bone turnover. J. Bone Miner. Res. 25, 1627–1636 (2010).
Rodriguez, L. et al. Expression and functional assessment of an alternatively spliced extracellular Ca2+-sensing receptor in growth plate chondrocytes. Endocrinology 146, 5294–5303 (2005).
Tu, Q. et al. Rescue of the skeletal phenotype in CaSR-deficient mice by transfer onto the Gcm2 null background. J. Clin. Invest. 111, 1029–1037 (2003).
Miao, D., He, B., Karaplis, A. C. & Goltzman, D. Parathyroid hormone is essential for normal fetal bone formation. J. Clin. Invest. 109, 1173–1182 (2001).
Miao, D. et al. Skeletal abnormalities in Pth-null mice are influenced by calcium. Endocrinology 145, 2046–2053 (2004).
Dvorak-Ewell, M. M. et al. Osteoblast extracellular Ca2+-sensing receptor regulates bone development, mineralization, and turnover. J. Bone Miner. Res. 26, 2935–2947 (2011).
Chang, W. et al. Expression and signal transduction of calcium-sensing receptors in cartilage and bone. Endocrinology 140, 5883–5893 (1999).
Dvorak, M. M. et al. Physiological changes in extracellular calcium concentration directly control osteoblast function in the absence of calciotropic hormones. Proc. Natl Acad. Sci. USA 101, 5140–5145 (2004).
Yamaguchi, T. et al. Mouse osteoblastic cell line (MC3T3-E1) expresses extracellular calcium (Ca2+)-sensing receptor and its agonists stimulate chemotaxis and proliferation of MC3T3-E1 cells. J. Bone Miner. Res. 13, 1530–1538 (1998).
Chattopadhyay, N. et al. Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145, 3451–3562 (2004).
Yamaguchi, T., Chattopadhyay, N., Kifor, O., Sanders, J. L. & Brown, E. M. Activation of p42/44 and p38 mitogen-activated protein kinases by extracellular calcium-sensing receptor agonists induces mitogenic responses in the mouse osteoblastic MC3T3-E1 cell line. Biochem. Biophys. Res. Commun. 279, 363–368 (2000).
Saidak, Z. & Marie, P. J. Strontium signalling: molecular mechanisms and therapeutic implications in osteoporosis. Pharmacol. Ther. 136, 216–226 (2012).
Yamauchi, M., Yamaguchi, T., Kaji, H., Sugimoto, T. & Chihara, K. Involvement of calcium-sensing receptor in osteoblastic differentiation of mouse MC3T3-E1 cells. Am. J. Physiol. Endocrinol. Metab. 288, E608–E616 (2005).
Chattopadhyay, N. et al. Mitogenic action of calcium-sensing receptor on rat calvarial osteoblasts. Endocrinology 145, 3451–3412 (2004).
Takaoka, S., Yamaguchi, T., Yano, S., Yamauchi, M. & Sugimoto, T. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast differentiation and mineralization. Horm. Metab. Res. 42, 627–631 (2010).
Brennan, T. C. et al. Osteoblasts play key roles in the mechanisms of action of strontium ranelate. Br. J. Pharmacol. 157, 1291–1300 (2009).
Atkins, G. J., Welldon, K. J., Halbout, P. & Findlay, D. M. Strontium ranelate treatment of human primary osteoblasts promotes and osteocyte-like phenotype while eliciting an osteoprotegerin response. Osteoporosis Int. 20, 653–664 (2009).
Fromigué, O. et al. Calcium-sensing receptor-dependent and receptor-independent activation of osteoblasts replication and survival by strontium ranelate. J. Cell. Mol. Med. 13, 2189–2199 (2009).
Pi, M., Chen, J., Zhu, W. & Quarles, L. D. Dominant negative effect of the extracellular domain of CASR. J. Receptor Ligand Channel Res. 3, 15–23 (2010).
Cao, G. et al. Parathyroid hormone contributes to regulating milk calcium content and modulates neonatal bone formation cooperatively with calcium. Endocrinology 150, 561–569 (2009).
Pi, M. et al. Identification of a novel extracellular cation-sensing G-protein-coupled receptor. J. Biol. Chem. 280, 40201–40209 (2005).
Pi, M. et al. GPRC6A null mice exhibit osteopenia, feminization and metabolic syndrome. PLoS ONE 3, e3858 (2008).
Wellendorph, P. et al. No evidence for a bone phenotype in GPRC6A knockout mice under normal physiological conditions. J. Mol. Endocrinol. 42, 215–223 (2009).
Shu, L. et al. The calcium-sensing receptor mediates bone turnover induced by dietary calcium and parathyroid hormone in neonates. J. Bone Miner. Res. 26, 1057–1071 (2011).
Xue, Y. et al. The calcium-sensing receptor complements parathyroid hormone-induced bone turnover in discrete skeletal compartments in mice. Am. J. Physiol. Endocrinol. Metab. 302, E841–E851 (2012).
House, M. G. et al. Expression of an extracellular calcium-sensing receptor in human and mouse bone marrow cells. J. Bone Miner. Res. 12, 1959–1970 (1997).
Kanatani, M., Sugimoto, T., Kanazawa, M., Yano, S. & Chihara, K. High extracellular calcium inhibits osteoclast-like cell formation by directly acting on the calcium-sensing receptor existing in osteoclast precursor cells. Biochem. Biophys. Res. Commun. 261, 144–148 (1999).
Kameda, T. et al. Calcium-sensing receptor in mature osteoclasts, which are bone resorbing cells. Biochem. Biophys. Res. Commun. 245, 419–427 (1998).
Datta, H. K., MacIntyre, I. & Zaidi, M. The effect of extracellular calcium elevation on morphology and function of isolated rat osteoclasts. Biosci. Rep. 9, 747–751 (1989).
Malgaroli, A., Meldolesi, J., Zallone, A. Z. & Teti, A. Control of cytosolic free calcium in rat and chicken osteoclasts. The role of extracellular calcium and calcitonin. J. Biol. Chem. 264, 14342–14347 (1989).
Moonga, B. S., Moss, D. W., Patchell, A. & Zaidi, M. Intracellular regulation of enzyme secretion from rat osteoclasts and evidence for a functional role in bone resorption. J. Physiol. 429, 29–45 (1990).
Zaidi, M. et al. Divalent cations mimic the inhibitory effect of extracellular ionized calcium on bone resorption by isolated rat osteoclasts: further evidence for a “calcium receptor”. J. Cell. Physiol. 149, 422–427 (1991).
Mentaverri, R. et al. The calcium-sensing receptor is directly involved in both osteoclast differentiation and apoptosis. FASEB J. 20, 2562–2564 (2006).
Shaloub, V. et al. In vitro studies with the calcimimetic, cinacalcet HCl, on normal human adult osteoblastic and osteoclastic cells. Crit. Rev. Eukaryot. Gene Expr. 13, 89–106 (2003).
Dvorak, M. M. et al. Constitutive activity of the osteoblast Ca2+-sensing receptor promotes loss of cancellous bone. Endocrinology 148, 3156–3163 (2007).
Lieben, L. et al. Normocalcemia is maintained in mice under conditions of malabsorption by vitamin D-induced inhibition of bone mineralization. J. Clin. Invest. 122, 1803–1815 (2012).
Brown, E. M. Extracellular Ca2+ signaling, regulation of parathyroid cell function, and role of Ca2+ and other ions as extracellular (first) messengers. Physiol. Rev. 71, 371–411 (1991).
Silver, I. A., Murrills, R. J. & Etherington, D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp. Cell Res. 175, 266–276 (1988).
Garner, S. C., Pi, M., Tu, Q. & Quarles, L. D. Rickets in calcium-sensing receptor-deficient mice: an unexpected skeletal phenotype. Endocrinology 142, 3996–4005 (2001).
Ho, C. et al. A mouse model of human familial hypocalciuric hypercalcemia and neonatal severe hyperparathyroidism. Nat. Genet. 11, 389–394 (1995).
Sun, W. et al. Alterations in phosphorus, calcium and PTHrP contribute to defects in dental and dental alveolar bone formation in calcium-sensing receptor-deficient mice. Development 137, 985–992 (2010).
Gopalakrishnan, R., Suttamanatwong, S., Carlson, S., Carlson, A. E. & Franceschi, R. T. Role of matrix Gla protein in parathyroid hormone inhibition of osteoblast mineralization. Cells Tissues Organs 181, 166–175 (2005).
Imanishi, Y. et al. Primary hyperparathyroidism caused by parathyroid-targeted expression of cyclin D1 in transgenic mice. J. Clin. Invest. 107, 1093–1102 (2001).
Parisien, M. et al. The histomorphometry of bone in primary hyperparathyroidism: preservation of cancellous bone structure. J. Clin. Endocrinol. Metab. 70, 930–938 (1990).
Wada, M. et al. NPS R-568 halts or reverses osteotis fibrosa in uremic rats. Kidney Int. 53, 448–453 (1998).
Henley, C. et al. The calcimimetic AMG 641 abrogates parathyroid hyperplasia, bone and vascular calcification abnormalities in uremic rats. Eur. J. Pharmacol. 616, 306–313 (2009).
Finch, J. L. et al. Effect of paricalcitol and cinacalcet on serum phosphate, FGF-23, and bone in rats with chronic kidney disease. Am. J. Physiol. Renal Physiol. 298, F1315–F1322 (2010).
Hendy, G. N., Guarnieri, V. & Canaff, L. Calcium-sensing receptor and associated diseases. Prog. Mol. Biol. Transl. Sci. 89, 31–95 (2009).
Hannan, F. M. & Thakker, R. V. Calcium-sensing receptor (CaSR) mutations and disorders of calcium, electrolyte and water metabolism. Best Pract. Res. Clin. Endocrinol. Metab. 27, 359–371 (2013).
Menko, F. H. et al. Familial benign hypercalcemia. Study of a large family. Quart. J. Med. 206, 120–140 (1983).
Kristiansen, J. H., Rødbro, P., Christiansen, C., Johansen, J. & Jensen, J. T. Familial hypocalciuric hypercalcemia. III: Bone mineral metabolism. Clin. Endocrinol. (Oxf.) 26, 713–716 (1987).
Jakobsen, N. F. et al. Increased trabecular volumetric bone mass density in familial hypocalciuric hypercalcemia (FHH) type 1: a cross-sectional study. Calcif. Tissue Int. 95, 141–152 (2014).
Abugassa, S., Nordenstrom, J. & Jähult, J. Bone mineral density in patients with familial hypocalciuric hypercalcemia (FHH). Eur. J. Surg. 158, 397–402 (1992).
Christensen, S. E. et al. Skeletal consequences of familial hypocalciuric hypercalcaemia vs. primary hyperparathyroidism. Clin. Endocrinol. 71, 798–807 (2009).
Law, W. M. Jr., Wahner, H. W. & Heath, H. 3rd. Bone mineral density and skeletal fractures in familial benign hypercalcemia (hypocalciuric). Mayo Clin. Proc. 59, 811–815 (1984).
Isaksen, T. et al. Forearm bone mineral density in familial hypocalciuric hypercalcemia and primary hyperparathyroidism: a comparative study. Calcif. Tissue Int. 89, 285–294 (2011).
Reh, C. M., Hendy, G. N., Cole, D. E. & Jeandron, D. D. Neonatal hyperparathyroidism with a heterozygous calcium-sensing receptor (CASR) R185Q mutation: clinical benefits from cinacalcet. J. Clin. Endocrinol. Metab. 96, E707–E712 (2011).
Wilhelm-Bals, A., Parvex, P., Magdelaine, C. & Girardin, E. Successful use of bisphosphonate and calcimimetic in neonatal severe primary hyperparathyroidism. Pediatrics 129, e812–e816 (2012).
Alon, U. S. & VandeVoorde, R. G. Beneficial effect of cinacalcet in a child with familial hypocalciuric hypercalcemia. Pediatr. Nephrol. 25, 1747–1750 (2010).
Theman, T. A. et al. PTH1–34 replacement therapy in a child with hypoparathyroidism caused by a sporadic calcium receptor mutation. J. Bone Miner. Res. 24, 964–973 (2009).
Peacock, M. et al. Cinacalcet hydrochloride maintains long-term normocalcemia in patients with primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 90, 135–141 (2005).
Peacock, M. et al. Cinacalcet treatment of primary hyperparathyroidism: biochemical and bone densitometric outcomes in a five-year study. J. Clin. Endocrinol. Metab. 94, 4860–4867 (2009).
Marotta, V. et al. Potential role of cinacalcet hydrochloride in sporadic primary hyperparathyroidism without surgery indication. Endocrine http://dx.doi.org/10.1007/s12020-014-0381–0.
Silverberg, S. J. et al. Increased bone mineral density after parathyroidectomy in primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 80, 729–734 (1995).
Christiansen, P. et al. Primary hyperparathyroidism: effect of parathyroidectomy on regional bone mineral density in Danish patients: a three-year follow-up study. Bone 25, 589–595 (1999).
Christiansen, P. et al. Primary hyperparathyroidism: short-term changes in bone remodeling and bone mineral density following parathyroidectomy. Bone 25, 237–244 (1999).
Moosgaard, B. et al. Vitamin D metabolites and skeletal consequences in primary hyperparathyroidism. Clin. Endocrinol. (Oxf.) 68, 707–715, (2008).
Leppla, D. C., Snyder, W. & Pak, C. Y. Sequential changes in bone density before and after parathyroidectomy in primary hyperparathyroidism. Invest. Radiol. 17, 604–606 (1982).
Ambrogini, E. et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J. Clin. Endocrinol. Metab. 92, 3114–3121 (2007).
Bollerslev, J. et al. Medical observation, compared with parathyroidectomy, for asymptomatic primary hyperparathyroidism: a prospective, randomized trial. J. Clin. Endocrinol. Metab. 92, 1687–1692 (2007).
Koumakis, E. et al. Bone mineral density evolution after successful parathyroidectomy in patients with normocalcemic primary hyperparathyroidism. J. Clin. Endocrinol. Metab. 98, 3213–3220 (2013).
Eller-Vainicher, C. et al. Factors associated with vertebral fracture risk in patients with primary hyperparathyroidism. Eur. J. Endocrinol. 171, 399–406 (2014).
Ballinger, A. E., Palmer, S. C., Nistor, I., Craig, J. C. & Strippoli, G. F. Calcimimetics for secondary hyperparathyroidism in chronic kidney disease patients. Cochrane Database of Systematic Reviews, Issue 12. Art. No.: CD006254. http://dx.doi.org/10.1002/14651858.CD006254.pub2.
Palmer, S. C. et al. Cinacalcet in patients with chronic kidney disease: a cumulative meta-analysis of randomized controlled trials. PLoS Med. 10, e1001436 (2013).
Jean, G. Chazot, C. & Charra, B. 12 months cinacalcet therapy in hemodialysis patients with secondary hyperparathyroidism: effect on bone markers. Clin. Nephrol. 68, 63–64 (2007).
Lien, Y. H., Silva, A. L. & Whittman, D. Effects of cinacalcet on bone mineral density in patients with secondary hyperparathyroidism. Nephrol. Dial. Transplant. 20, 1232–1237 (2005).
Malluche, H. H. et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin. Nephrol. 69, 269–278 (2008).
Behets, G. J. et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. http://dx.doi.org/10.1038/ki.2014.349.
Yajima, A., Akizawa, T., Tsukamoto, Y., Kurihara, S. & Ito, A. Impact of cinacalcet hydrochloride on bone histology in patients with secondary hyperparathyroidism. Ther. Apher. Dial. 1, S38–S43 (2008).
Fitzpatrick, L. A. et al. Ronacaleret, a calcium-sensing receptor antagonist, increases trabecular but not cortical bone in postmenopausal women. J. Bone Miner. Res. 27, 255–262 (2012).
Halse, J. et al. A phase 2, randomized, placebo-controlled, dose-ranging study of the calcium-sensing receptor antagonist MK-5442 in the treatment of postmenopausal women with osteoporosis. J. Clin. Endocrinol. Metab. 99, E2207–E2215 (2014).
Nemeth, E. F. & Shoback, D. Calcimimetic and calcilytic drugs for treating bone and mineral-related disorders. Best Pract. Res. Clin. Endocrinol. Metab. 27, 373–384 (2013).
Acknowledgements
The authors acknowledge the Canadian Institutes of Health Research for supporting their work.
Author information
Authors and Affiliations
Contributions
D.G. and G.N.H. researched data for the article, provided substantial contributions to discussions of the content, contributed equally to writing the article, and to review and/or editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Goltzman, D., Hendy, G. The calcium-sensing receptor in bone—mechanistic and therapeutic insights. Nat Rev Endocrinol 11, 298–307 (2015). https://doi.org/10.1038/nrendo.2015.30
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.30
This article is cited by
-
Calcium-sensing receptor and CPAP-induced neonatal airway hyperreactivity in mice
Pediatric Research (2022)
-
Skeletal toxicity resulting from exposure of growing male rats to coplanar PCB 126 is associated with disruption of calcium homeostasis and the GH-IGF-1 axis and direct effects on bone formation
Archives of Toxicology (2020)
-
The calcium-sensing receptor in physiology and in calcitropic and noncalcitropic diseases
Nature Reviews Endocrinology (2019)
-
Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells via increasing osteopontin expression
Experimental & Molecular Medicine (2018)
-
Comprehensive in vitro and in vivo studies of novel melt-derived Nb-substituted 45S5 bioglass reveal its enhanced bioactive properties for bone healing
Scientific Reports (2018)