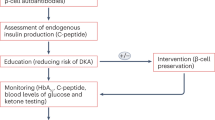
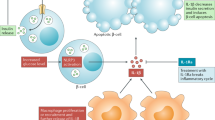
Key Points
-
As several mechanisms lead to β-cell failure in type 1 diabetes mellitus (T1DM), strategies combining different molecules could target different pathways involved in the pathogenesis of T1DM
-
Different combination therapies have been tested in preclinical studies with encouraging results, but results from clinical trials have not matched expectations
-
Heterogeneity in the age of patients with T1DM and the low rate of human β-cell regeneration are possible explanations for the negative results of clinical trials that should be addressed in future studies
-
Proton pump inhibitors and incretin-based agents can stimulate β-cell regeneration, so future trials in patients with T1DM should investigate their potential in combination with immunomodulatory compounds
-
The issue of patient heterogeneity can be addressed by designing trials evaluating the relative effectiveness of interventions in specific subgroups of patients
Abstract
Immunotherapies for type 1 diabetes mellitus (T1DM) have been the focus of intense basic and clinical research over the past few decades. Restoring β-cell function is the ultimate goal of intervention trials that target the immune system in T1DM. In an attempt to achieve this aim, different combination therapies have been proposed over the past few years that are based on treatments tackling the various mechanisms involved in the destruction of β cells. The results of clinical trials have not matched expectations based on the positive results from preclinical studies. The heterogeneity of T1DM might explain the negative results obtained, but previous trials have not addressed this issue. However, novel promising combination therapies are being developed, including those that couple immunomodulators with drugs that stimulate β-cell regeneration in order to restore normoglycaemia. This strategy is an encouraging one to pursue the goal of finding a cure for T1DM. This Review summarizes the available data about combination immunotherapies in T1DM, particularly addressing their clinical importance. The available data supporting the use of registered drugs, such as proton pump inhibitors and incretin-based agents, that have been shown to induce β-cell regeneration will also be discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Levetan, C., Pozzilli, P., Jovanovic, L. & Schatz, D. Proposal for generating new β cells in a muted immune environment for type 1 diabetes. Diabetes Metab. Res. Rev. 29, 604–606 (2013).
Baekkeskov, S. et al. Identification of the 64K autoantigen in insulin-dependent diabetes as the GABA-synthesizing enzyme glutamic acid decarboxylase. Nature 347, 151–156 (1990).
Mannering, S. I. et al. The insulin A-chain epitope recognized by human T cells is posttranslationally modified. J. Exp. Med. 202, 1191–1197 (2005).
Stadinski, B. D. et al. Chromogranin A is an autoantigen in type 1 diabetes. Nat. Immunol. 11, 225–231 (2010).
Strollo, R. et al. HLA-dependent autoantibodies against post-translationally modified collagen type II in type 1 diabetes mellitus. Diabetologia 56, 563–572 (2013).
Van Lummel, M. et al. Posttranslational modification of HLA-DQ binding islet autoantigens in type 1 diabetes. Diabetes 63, 237–247 (2014).
Rondas, D. et al. Citrullinated glucose-regulated protein 78 is an autoantigen in type 1 diabetes. Diabetes 64, 573–586 (2015).
Pescovitz, M. D. et al. Rituximab, B-lymphocyte depletion, and preservation of β-cell function. N. Engl. J. Med. 361, 2143–2152 (2009).
Orban, T. et al. Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: a randomised, double-blind, placebo-controlled trial. Lancet 378, 412–419 (2011).
Harrison, L. C. et al. Pancreatic β-cell function and immune responses to insulin after administration of intranasal insulin to humans at risk for type 1 diabetes. Diabetes Care 27, 2348–2355 (2004).
Walter, M., Philotheou, A., Bonnici, F., Ziegler, A.-G. & Jimenez, R. No effect of the altered peptide ligand NBI-6024 on β-cell residual function and insulin needs in new-onset type 1 diabetes. Diabetes Care 32, 2036–2040 (2009).
Ludvigsson, J. et al. GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N. Engl. J. Med. 366, 433–442 (2012).
Suarez-Pinzon, W. L., Lakey, J. R. T. & Rabinovitch, A. Combination therapy with glucagon-like peptide-1 and gastrin induces β-cell neogenesis from pancreatic duct cells in human islets transplanted in immunodeficient diabetic mice. Cell Transplant. 17, 631–640 (2008).
Griffin, K. J., Thompson, P. A., Gottschalk, M., Kyllo, J. H. & Rabinovitch, A. Combination therapy with sitagliptin and lansoprazole in patients with recent-onset type 1 diabetes (REPAIR-T1D): 12-month results of a multicentre, randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 2, 710–718 (2014).
Ferrannini, E. The stunned β cell: a brief history. Cell Metab. 11, 349–352 (2010).
Lebastchi, J. & Herold, K. C. Immunologic and metabolic biomarkers of β-cell destruction in the diagnosis of type 1 diabetes. Cold Spring Harb. Perspect. Med. 2, a007708 (2012).
Atkinson, M. A., Eisenbarth, G. S. & Michels, A. W. Type 1 diabetes. Lancet 383, 69–82 (2014).
Chianelli, M. et al. Pancreatic scintigraphy with 99mTc-interleukin-2 at diagnosis of type 1 diabetes and after 1 year of nicotinamide therapy. Diabetes Metab. Res. Rev. 24, 115–122 (2008).
Ueberberg, S. et al. Generation of novel single-chain antibodies by phage-display technology to direct imaging agents highly selective to pancreatic β- or α-cells in vivo. Diabetes 58, 2324–2334 (2009).
Goland, R. et al. 11C-dihydrotetrabenazine PET of the pancreas in subjects with long-standing type 1 diabetes and in healthy controls. J. Nucl. Med. 50, 382–9 (2009).
Robertson, R. P. Estimation of β-cell mass by metabolic tests: necessary, but how sufficient? Diabetes 56, 2420–2424 (2007).
Cernea, S. et al. Challenges in developing endpoints for type 1 diabetes intervention studies. Diabetes Metab. Res. Rev. 25, 694–704 (2009).
Block, M. B., Rosenfield, R. L., Mako, M. E., Steiner, D. F. & Rubenstein, A. H. Sequential changes in β-cell function in insulin-treated diabetic patients assessed by C-peptide immunoreactivity. N. Engl. J. Med. 288, 1144–1148 (1973).
Palmer, J. P. et al. C-peptide is the appropriate outcome measure for type 1 diabetes clinical trials to preserve β-cell function. ADA workshop report. Diabetes 53, 250–264 (2004).
Greenbaum, C. J. et al. Mixed-meal tolerance test versus glucagon stimulation test for the assessment of β-cell function in therapeutic trials in type 1 diabetes. Diabetes Care 31, 1966–1971 (2008).
Pozzilli, P. et al. Evaluation of long-term treatment effect in a type 1 diabetes intervention trial: differences after stimulation with glucagon or a mixed meal. Diabetes Care 37, 1384–1391 (2014).
Kaas, A. et al. Proinsulin, GLP-1, and glucagon are associated with partial remission in children and adolescents with newly diagnosed type 1 diabetes. Pediatr. Diabetes 13, 51–58 (2012).
[No authors listed] Effects of age, duration and treatment of insulin-dependent diabetes mellitus on residual β-cell function: observations during eligibility testing for the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. J. Clin. Endocrinol. Metab. 65, 30–36 (1987).
[No authors listed] Effect of intensive therapy on residual β-cell function in patients with type 1 diabetes in the diabetes control and complications trial. A randomized, controlled trial. The Diabetes Control and Complications Trial Research Group. Ann. Intern. Med. 128, 517–523 (1998).
Sosenko, J. M. et al. Glucose and C-peptide changes in the perionset period of type 1 diabetes in the Diabetes Prevention Trial-Type 1. Diabetes Care 31, 2188–2192 (2008).
Pozzilli, P. et al. Metabolic and immune parameters at clinical onset of insulin-dependent diabetes: a population-based study. IMDIAB Study Group. Immunotherapy Diabetes. Metabolism 47, 1205–1210 (1998).
Pozzilli, P. et al. Is the process of β-cell destruction in type 1 diabetes at time of diagnosis more extensive in females than in males? Eur. J. Endocrinol. 145, 757–761 (2001).
Dabelea, D. et al. Clinical evolution of β-cell function in youth with diabetes: the SEARCH for Diabetes in Youth study. Diabetologia 55, 3359–3368 (2012).
Greenbaum, C. J. et al. Fall in C-peptide during first 2 years from diagnosis: evidence of at least two distinct phases from composite Type 1 Diabetes TrialNet data. Diabetes 61, 2066–2073 (2012).
Barker, A. et al. Age-dependent decline of β-cell function in type 1 diabetes after diagnosis: a multi-centre longitudinal study. Diabetes. Obes. Metab. 16, 262–267 (2014).
Törn, C. et al. Prognostic factors for the course of β-cell function in autoimmune diabetes. J. Clin. Endocrinol. Metab. 85, 4619–4623 (2000).
Picardi, A. et al. Metabolic factors affecting residual β-cell function assessed by C-peptide secretion in patients with newly diagnosed type 1 diabetes. Horm. Metab. Res. 38, 668–672 (2006).
Weets, I. et al. Sex- and season-dependent differences in C-peptide levels at diagnosis of immune-mediated type 1 diabetes. Diabetologia 49, 1158–1162 (2006).
Petrone, A. et al. Residual insulin secretion at diagnosis of type 1 diabetes is independently associated with both, age of onset and HLA genotype. Diabetes Metab. Res. Rev. 21, 271–275 (2005).
Pozzilli, P. Type 1 diabetes mellitus in 2011: Heterogeneity of T1DM raises questions for therapy. Nat. Rev. Endocrinol. 8, 78–80 (2012).
Buzzetti, R. et al. C-peptide response and HLA genotypes in subjects with recent-onset type 1 diabetes after immunotherapy with DiaPep277: an exploratory study. Diabetes 60, 3067–3072 (2011).
Dor, Y., Brown, J., Martinez, O. I. & Melton, D. A. Adult pancreatic β-cells are formed by self-duplication rather than stem-cell differentiation. Nature 429, 41–46 (2004).
Meier, J. J., Bhushan, A., Butler, A. E., Rizza, R. A. & Butler, P. C. Sustained β cell apoptosis in patients with long-standing type 1 diabetes: indirect evidence for islet regeneration? Diabetologia 48, 2221–2228 (2005).
Akerblom, H. K. et al. The case for elimination of cow's milk in early infancy in the prevention of type 1 diabetes: the Finnish experience. Diabetes Metab. Rev. 9, 269–278 (1993).
Roep, B. O. et al. Molecular mimicry in type 1 diabetes: immune cross-reactivity between islet autoantigen and human cytomegalovirus but not Coxsackie virus. Ann. NY Acad. Sci. 958, 163–165 (2002).
Afonso, G. & Mallone, R. Infectious triggers in type 1 diabetes: is there a case for epitope mimicry? Diabetes. Obes. Metab. 15 (Suppl. 3), 82–88 (2013).
Brugman, S. et al. Antibiotic treatment partially protects against type 1 diabetes in the Bio-Breeding diabetes-prone rat. Is the gut flora involved in the development of type 1 diabetes? Diabetologia 49, 2105–2108 (2006).
Fousteri, G., von Herrath, M. & Bresson, D. Mucosal exposure to antigen: cause or cure of type 1 diabetes? Curr. Diab. Rep. 7, 91–98 (2007).
Richardson, S. J., Morgan, N. G. & Foulis, A. K. Pancreatic pathology in type 1 diabetes mellitus. Endocr. Pathol. 25, 80–92 (2014).
Chen, Z., Herman, A. E., Matos, M., Mathis, D. & Benoist, C. Where CD4+CD25+ T reg cells impinge on autoimmune diabetes. J. Exp. Med. 202, 1387–1397 (2005).
Moran, A. et al. Interleukin-1 antagonism in type 1 diabetes of recent onset: two multicentre, randomised, double-blind, placebo-controlled trials. Lancet 381, 1905–1915 (2013).
Peakman, M. & von Herrath, M. Antigen-specific immunotherapy for type 1 diabetes: maximizing the potential. Diabetes 59, 2087–2093 (2010).
Herold, K. C. et al. Teplizumab treatment may improve C-peptide responses in participants with type 1 diabetes after the new-onset period: a randomised controlled trial. Diabetologia 56, 391–400 (2013).
Herold, K. C. et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes 62, 3766–3774 (2013).
Aronson, R. et al. Low-dose otelixizumab anti-CD3 monoclonal antibody DEFEND-1 study: results of the randomized phase III study in recent-onset human type 1 diabetes. Diabetes Care 37, 2746–2754 (2014).
Gitelman, S. E. et al. Antithymocyte globulin treatment for patients with recent-onset type 1 diabetes: 12-month results of a randomised, placebo-controlled, phase 2 trial. Lancet Diabetes Endocrinol. 1, 306–316 (2013).
Rigby, M. R. et al. Targeting of memory T cells with alefacept in new-onset type 1 diabetes (T1DAL study): 12 month results of a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Diabetes Endocrinol. 1, 284–294 (2013).
Sherry, N. A. et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of β cells. Endocrinology 148, 5136–5144 (2007).
Ablamunits, V. et al. Synergistic reversal of type 1 diabetes in NOD mice with anti-CD3 and interleukin-1 blockade: evidence of improved immune regulation. Diabetes 61, 145–154 (2012).
Perl, S. et al. Addition of rapamycin to anti-CD3 antibody improves long-term glycaemia control in diabetic NOD mice. PLoS ONE 8, e67189 (2013).
Matthews, J. B., Staeva, T. P., Bernstein, P. L., Peakman, M. & von Herrath, M. Developing combination immunotherapies for type 1 diabetes: recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin. Exp. Immunol. 160, 176–184 (2010).
Long, S. A. et al. Rapamycin/IL-2 combination therapy in patients with type 1 diabetes augments Tregs yet transiently impairs β-cell function. Diabetes 61, 2340–2348 (2012).
Gottlieb, P. A. et al. Failure to preserve β-cell function with mycophenolate mofetil and daclizumab combined therapy in patients with new- onset type 1 diabetes. Diabetes Care 33, 826–832 (2010).
Pozzilli, P. & Di Mario, U. Autoimmune diabetes not requiring insulin at diagnosis (latent autoimmune diabetes of the adult): definition, characterization, and potential prevention. Diabetes Care 24, 1460–1467 (2001).
Mollo, A. et al. Latent autoimmune diabetes in adults is perched between type 1 and type 2: evidence from adults in one region of Spain. Diabetes. Metab. Res. Rev. 29, 446–451 (2013).
Gruessner, R. W. G. & Gruessner, A. C. The current state of pancreas transplantation. Nat. Rev. Endocrinol. 9, 555–562 (2013).
Bruni, A., Gala-Lopez, B., Pepper, A. R., Abualhassan, N. S. & Shapiro, A. J. Islet cell transplantation for the treatment of type 1 diabetes: recent advances and future challenges. Diabetes Metab. Syndr. Obes. 7, 211–223 (2014).
Aguayo-Mazzucato, C. & Bonner-Weir, S. Stem cell therapy for type 1 diabetes mellitus. Nat. Rev. Endocrinol. 6, 139–148 (2010).
Orlando, G. et al. Cell replacement strategies aimed at reconstitution of the β-cell compartment in type 1 diabetes. Diabetes 63, 1433–1444 (2014).
Bouwens, L., Houbracken, I. & Mfopou, J. K. The use of stem cells for pancreatic regeneration in diabetes mellitus. Nat. Rev. Endocrinol. 9, 598–606 (2013).
Baeyens, L. et al. In vitro generation of insulin-producing β cells from adult exocrine pancreatic cells. Diabetologia 48, 49–57 (2005).
Collombat, P. et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell 138, 449–462 (2009).
Wu, F. et al. TNF-like weak inducer of apoptosis (TWEAK) promotes β-cell neogenesis from pancreatic ductal epithelium in adult mice. PLoS ONE 8, e72132 (2013).
Avolio, F. et al. From pancreas morphogenesis to β-cell regeneration. Curr. Top. Dev. Biol. 106, 217–238 (2013).
Wang, R. N., Rehfeld, J. F., Nielsen, F. C. & Klöppel, G. Expression of gastrin and transforming growth factor-α during duct to islet cell differentiation in the pancreas of duct-ligated adult rats. Diabetologia 40, 887–893 (1997).
Boj-Carceller, D. Proton pump inhibitors: impact on glucose metabolism. Endocrine 43, 22–32 (2013).
Mefford, I. N. & Wade, E. U. Proton pump inhibitors as a treatment method for type II diabetes. Med. Hypotheses 73, 29–32 (2009).
Boj-Carceller, D. et al. Are proton pump inhibitors a new antidiabetic drug? A cross sectional study. World J. Diabetes 2, 217–220 (2011).
Hove, K. D. et al. Treatment with a proton pump inhibitor improves glycaemic control in type 2 diabetic patients—a retrospective analysis. Diabetes Res. Clin. Pract. 90, e72–e74 (2010).
Crouch, M. A., Mefford, I. N. & Wade, E. U. Proton pump inhibitor therapy associated with lower glycosylated hemoglobin levels in type 2 diabetes. J. Am. Board Fam. Med. 25, 50–54 (2012).
Guglielmi, C., Secchi, C., Maddaloni, E., Manfrini, S. & Pozzilli, P. Effects of Treatment with Proton Pump Inhibitors in Metabolic Control in Patients with type 2 Diabetes [abstract 2425PO]. Diabetes 63 (Suppl. 1), A615 (2014).
Singh, P. K. et al. Pantoprazole improves glycemic control in type 2 diabetes: a randomized, double-blind, placebo-controlled trial. J. Clin. Endocrinol. Metab. 97, E2105–E2108 (2012).
Hove, K. D. et al. Effects of 12 weeks' treatment with a proton pump inhibitor on insulin secretion, glucose metabolism and markers of cardiovascular risk in patients with type 2 diabetes: a randomised double-blind prospective placebo-controlled study. Diabetologia 56, 22–30 (2013).
Pettus, J., Hirsch, I. & Edelman, S. GLP-1 agonists in type 1 diabetes. Clin. Immunol. 149, 317–323 (2013).
Farilla, L. et al. Glucagon-like peptide 1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology 144, 5149–5158 (2003).
Bunck, M. C. et al. Effects of exenatide on measures of β-cell function after 3 years in metformin-treated patients with type 2 diabetes. Diabetes Care 34, 2041–2047 (2011).
Poucher, S. M. et al. Effects of saxagliptin and sitagliptin on glycaemic control and pancreatic β-cell mass in a streptozotocin-induced mouse model of type 2 diabetes. Diabetes Obes. Metab. 14, 918–926 (2012).
Liang, J., Leung, K. K., Lam, S. Y. & Leung, P. S. Combined treatment with a dipeptidyl peptidase-IV inhibitor (sitagliptin) and an angiotensin II type 1 receptor blocker (losartan) promotes islet regeneration via enhanced differentiation of pancreatic progenitor cells. Diabetes Obes. Metab. 14, 842–851 (2012).
Pospisilik, J. A. et al. Dipeptidyl peptidase IV inhibitor treatment stimulates β-cell survival and islet neogenesis in streptozotocin-induced diabetic rats. Diabetes 52, 741–750 (2003).
Jelsing, J., Vrang, N., van Witteloostuijn, S. B., Mark, M. & Klein, T. The DPP4 inhibitor linagliptin delays the onset of diabetes and preserves β-cell mass in non-obese diabetic mice. J. Endocrinol. 214, 381–387 (2012).
Kim, D.-H., Lee, J.-C., Lee, M.-K., Kim, K.-W. & Lee, M.-S. Treatment of autoimmune diabetes in NOD mice by Toll-like receptor 2 tolerance in conjunction with dipeptidyl peptidase 4 inhibition. Diabetologia 55, 3308–3317 (2012).
Cai, X., Han, X., Luo, Y. & Ji, L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on β-cell function in Asian and Caucasian type 2 diabetes mellitus patients: A meta-analysis. J. Diabetes http://dx.doi.org/10.1111/1753-0407.12196.
Zhao, Y., Yang, L. & Zhou, Z. Dipeptidyl peptidase-4 inhibitors: multitarget drugs, not only antidiabetes drugs. J. Diabetes 6, 21–29 (2014).
Tian, L. et al. Reversal of new-onset diabetes through modulating inflammation and stimulating β-cell replication in nonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology 151, 3049–3060 (2010).
Zhao, Y., Yang, L., Wang, X. & Zhou, Z. The new insights of DPP-4 inhibitors: their potential immune modulatory function in autoimmune diabetes. Diabetes Metab. Res. Rev. 30, 646–653 (2014).
Goodwin, S. R. et al. Dipeptidyl peptidase IV inhibition does not adversely affect immune or virological status in HIV infected men and women: a pilot safety study. J. Clin. Endocrinol. Metab. 98, 743–751 (2013).
Ellis, S. L. et al. Effect of sitagliptin on glucose control in adult patients with type 1 diabetes: a pilot, double-blind, randomized, crossover trial. Diabet. Med. 28, 1176–1181 (2011).
Garg, S. K. et al. Effect of sitagliptin on post-prandial glucagon and GLP-1 levels in patients with type 1 diabetes: investigator-initiated, double-blind, randomized, placebo-controlled trial. Endocr. Pract. 19, 19–28 (2013).
Zhao, Y. et al. Dipeptidyl peptidase 4 inhibitor sitagliptin maintains β-cell function in patients with recent-onset latent autoimmune diabetes in adults: one year prospective study. J. Clin. Endocrinol. Metab. 99, E876–E880 (2014).
Johansen, O. E. et al. C-peptide levels in latent autoimmune diabetes in adults treated with linagliptin versus glimepiride: exploratory results from a 2-year double-blind, randomized, controlled study. Diabetes Care 37, e11–e12 (2014).
Pozzilli, P., Buzzetti, R., Frederich, R., Iqbal, N. & Hirshberg, B. Saxagliptin increases β-cell function and improves HOMA index in patients with latent autoimmune diabetes in adults [152-OR]. Diabetes 63, A41 (2014).
Ansarullah et al. Stimulating β-cell regeneration by combining a GPR119 agonist with a DPP-IV inhibitor. PLoS ONE 8, e53345 (2013).
Suarez-Pinzon, W. L., Cembrowski, G. S. & Rabinovitch, A. Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor restores normoglycaemia in non-obese diabetic mice. Diabetologia 52, 1680–1682 (2009).
Suarez-Pinzon, W. L. et al. Combination therapy with glucagon-like peptide-1 and gastrin restores normoglycemia in diabetic NOD mice. Diabetes 57, 3281–3288 (2008).
Suarez-Pinzon, W. L. & Rabinovitch, A. Combination therapy with a dipeptidyl peptidase-4 inhibitor and a proton pump inhibitor induces β-cell neogenesis from adult human pancreatic duct cells implanted in immunodeficient mice. Cell Transplant. 20, 1343–1349 (2011).
World Health Organization. Consolidated Guidelines on the use of antiretroviral drugs for treating and preventing HIV Infection [online], (2013).
Maddaloni, E. & Pozzilli, P. SMART diabetes: the way to go (Safe and Multifactorial Approach to reduce the Risk for Therapy in diabetes). Endocrine 46, 3–5 (2014).
Yacoub, T. G. Application of clinical judgment and guidelines to achieving glycemic goals in type 2 diabetes: focus on pharmacologic therapy. Postgrad. Med. 126, 95–106 (2014).
Acknowledgements
The authors acknowledge V. Greto, PhD student, for her invaluable help in searching PubMed for data for this Review.
Author information
Authors and Affiliations
Contributions
P.P. and E.M. researched data and wrote the manuscript. R.B. contributed to the discussion, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
P.P. has received honoraria and travel grants from Astra Zeneca, Eli Lilly, Merck Sharp & Dohme, Novartis, Takeda and Sanofi. R.B. has received honoraria from Astra Zeneca, Eli Lilly, Novartis, Novo Nordisk and Sanofi. E.M. declares no competing interests.
PowerPoint slides
Rights and permissions
About this article
Cite this article
Pozzilli, P., Maddaloni, E. & Buzzetti, R. Combination immunotherapies for type 1 diabetes mellitus. Nat Rev Endocrinol 11, 289–297 (2015). https://doi.org/10.1038/nrendo.2015.8
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2015.8
This article is cited by
-
Targeting protein phosphatases for the treatment of inflammation-related diseases: From signaling to therapy
Signal Transduction and Targeted Therapy (2022)
-
Adult-onset autoimmune diabetes
Nature Reviews Disease Primers (2022)
-
The association between overweight/obesity and double diabetes in adults with type 1 diabetes; a cross-sectional study
BMC Endocrine Disorders (2021)
-
Remission of autoimmune diabetes by anti-TCR combination therapies with anti-IL-17A or/and anti-IL-6 in the IDDM rat model of type 1 diabetes
BMC Medicine (2020)
-
Glucose-lowering and hypolipidemic activities of polysaccharides from Cordyceps taii in streptozotocin-induced diabetic mice
BMC Complementary and Alternative Medicine (2019)