Key Points
-
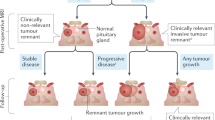
The WHO categorizes pituitary tumours as typical adenomas, atypical adenomas and pituitary carcinomas, although this classification does not cover the entire spectrum of histopathological findings or clinical behaviour
-
Pituitary adenomas are generally benign, but some display high rates of recurrence and/or resistance to conventional therapies—and are clinically defined as aggressive adenomas
-
Aggressive pituitary adenomas seem to represent a distinct entity requiring strict follow-up and early multimodal treatment, nonetheless, specific morphological, radiological and molecular diagnostic criteria should be identified
-
Pituitary carcinomas, by definition, are diagnosed only by demonstration of craniospinal dissemination or systemic metastases, although not all show cytological features of malignancy
-
Unlike pituitary carcinomas, aggressive pituitary adenomas do not give rise to metastases, but the two classes can share some histological features
-
Temozolomide might be useful in treating aggressive pituitary adenomas: anti-VEGF therapy, mTOR and tyrosine kinase inhibitors can also be used in selected patients
Abstract
The WHO categorizes pituitary tumours as typical adenomas, atypical adenomas and pituitary carcinomas, with typical adenomas constituting the major class. However, the WHO classification does not provide an accurate correlation between histopathological findings and clinical behaviour. Tumours lacking typical histological features are classified as atypical, but not all are clinically atypical or exhibit aggressive behaviour. Pituitary carcinomas, by definition, have craniospinal or systemic metastases, although not all display classical cytological features of malignancy. Aggressive pituitary adenomas, defined from a clinical perspective, have earlier and more frequent recurrences and can be resistant to conventional treatments. Specific biomarkers have not yet been identified that can distinguish between clinically aggressive and nonaggressive pituitary adenomas, although the antigen Ki-67 proliferation index might be of value. This Review highlights the need to develop new biomarkers to facilitate the early detection of clinically aggressive pituitary adenomas and discusses emerging markers that hold promise for their identification. Defining aggressiveness is of crucial importance for improving the management of patients by enhancing prognostic predictions and effectiveness of treatment. New drugs, such as temozolomide, have potential use in the management of these patients; anti-VEGF therapy, mTOR and tyrosine kinase inhibitors are also potentially useful in managing selected patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Asa, S. L. & Ezzat, S. The pathogenesis of pituitary tumors. Annu. Rev. Pathol. 4, 97–126 (2009).
Aflorei, E. D. & Korbonits, M. Epidemiology and etiopathogenesis of pituitary adenomas. J. Neurooncol. http://dx.doi.org/10.1007/s11060-013-1354–1355.
Scheithauer, B. W., Kovacs, K. T., Laws, E. R. Jr & Randall, R. V. Pathology of invasive pituitary tumors with special reference to functional classification. J. Neurosurg. 65, 733–744 (1986).
Thapar, K. et al. Proliferative activity and invasiveness among pituitary adenomas and carcinomas: an analysis using the MIB-1 antibody. Neurosurgery 38, 99–106 (1996).
Meij, B. P., Lopes, M. B., Ellegala, D. B., Alden, T. D. & Laws, E. R. Jr. The long-term significance of microscopic dural invasion in 354 patients with pituitary adenomas treated with transsphenoidal surgery. J. Neurosurg. 96, 195–208 (2002).
Kaltsas, G. A. et al. Diagnosis and management of pituitary carcinomas. J. Clin. Endocr. Metab. 90, 3089–3099 (2005).
Buchfelder, M. Management of aggressive pituitary adenomas: current treatment strategies. Pituitary 12, 256–260 (2009).
McCormack, A. I., Wass, J. A. & Grossman, A. B. Aggressive pituitary tumours: the role of temozolomide and the assessment of MGMT status. Eur. J. Clin. Invest. 41, 1133–1148 (2011).
Raverot, G. et al. Pituitary carcinomas and aggressive pituitary tumours: merits and pitfalls of temozolomide treatment. Clin. Endocrinol. (Oxf.) 76, 769–775 (2012).
Lloyd, R. V. et al. in Pathology and Genetics of Tumours of Endocrine Organs (eds DeLellis, R. A. et al.) 10–13 (IARC Press, 2004).
Saeger, W. et al. Pathohistological classification of pituitary tumors: 10 years of experience with the German Pituitary Tumor Registry. Eur. J. Endocrinol. 156, 203–216 (2007).
Zada, G. et al. Atypical pituitary adenomas: incidence, clinical characteristics, and implications. J. Neurosurg. 114, 336–344 (2011).
Scheithauer, B. W. et al. in Pituitary Carcinoma (eds DeLellis, R. A. et al.). 36–39 (IARC Press, 2004).
Melmed, S. Pathogenesis of pituitary tumors. Nat. Rev. Endocrinol. 7, 257–266 (2011).
Hardy, J. Transsphenoidal microsurgery of the normal and pathological pituitary. Clin. Neurosurg. 16, 185–217 (1969).
Campero, A., Martins, C., Yasuda, A. & Rhoton, A. L. Jr. Microsurgical anatomy of the diaphragm sellae and its role in directing the pattern of growth of pituitary adenomas. Neurosurgery 62, 717–723 (2008).
Di Ieva, A. et al. The subdiaphragmatic cistern: historic and radioanatomic findings. Acta Neurochir. (Wien) 154, 667–674 (2012).
Knosp, E., Steiner, E., Kitz, K. & Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: a magnetic resonance imaging classification compared with surgical findings. Neurosurgery 33, 610–617 (1993).
Cusimano, M. D. et al. Outcomes of surgically treated giant pituitary tumours. Can. J. Neurol. Sci. 39, 446–457 (2012).
Delgrange, E., Trouillas, J., Maiter, D., Donckier, J. & Tourniaire, J. Sex-related difference in the growth of prolactinomas: a clinical and proliferation marker study. J. Clin. Endocrinol. Metab. 82, 2012–2107 (1997).
Heaney, A. P. Clinical review: pituitary carcinoma: difficult diagnosis and treatment. J. Clin. Endocrinol. Metab. 96, 3649–3660 (2011).
Delgrange, E., Sassolas, G., Perin, G. & Trouillas, J. M. Clinical and histological correlations in prolactinomas, with specific reference to bromocriptine resistence. Acta Neurochir. (Wien.) 147, 4721–4727 (2005).
Colao, A., Grasso, L. F., Pivonello, R. & Lombardi, G. Therapy of aggressive pituitary tumors. Expert Opin. Pharmacother. 12, 1561–1570 (2011).
George, D. H. et al Crooke's cell adenoma of the pituitary: an aggressive variant of corticotroph adenoma. Am. J. Surg. Pathol. 27, 1330–1336 (2003).
Kovacs, K. et al. Prognostic indicators in an aggressive pituitary Crooke's cell adenoma. Can. J. Neurol. Sci. 32, 540–545 (2005).
Crooke, A. A change in the basophil cells of the pituitary gland common to conditions which exhibit the syndrome attributed to basophil adenoma. J. Pathol. Bacteriol. 41, 339–349 (1935).
Scheithauer, B. W. et al. Clinically silent corticotroph tumors of the pituitary gland. Neurosurgery 47, 723–729 (2000).
Jahangiri, A. et al. A comprehensive long-term retrospective analysis of silent corticotrophic adenomas versus hormone-negative adenomas. Neurosurgery 73, 8–17 (2013).
Asa, S. L., Ezzat, S., Watson, R. E., Lindell, E. P. & Horvath, E. in Tumors of Endocrine Organs (eds DeLellis, R. et al.) 30–32 (IARC Press, 2004).
Mete, O., Ezzat, S. & Asa, S. L. Biomarkers of aggressive pituitary adenomas. J. Mol. Endocrinol. 49, R69–R78 (2012).
Mete, O. & Asa, S. L. Clinicopathological correlations in pituitary adenomas. Brain Pathology 22, 443–453 (2012).
Mete, O. & Asa, S. L. Therapeutic implications of accurate classification of pituitary adenomas. Semin. Diagn. Pathol. 30, 158–164 (2013).
Batisse, M. et al. Aggressive silent GH pituitary tumor resistant to multiple treatments, including temozolomide. Cancer Invest. 31, 190–196 (2013).
Vieira Neto, L. et al. The role of temozolomide in the treatment of a patient with a pure silent pituitary somatotroph carcinoma. Endocr. Pract. 19, e145–e149 (2013).
Asa, S. L. et al. A growth hormone receptor mutation impairs growth hormone autofeedback signaling in pituitary tumors. Cancer Res. 67, 7505–7511 (2007).
Sano, T., Ohshima, T. & Yamada, S. Expression of glycoprotein hormones and intracytoplasmic distribution of cytokeratin in growth hormone-producing pituitary adenomas. Pathol. Res. Pract. 187, 530–533 (1991).
Hagiwara, A. et al. Comparison of growth hormone-producing and non-growth hormone-producing pituitary adenomas: imaging characteristics and pathologic correlation. Radiology 228, 533–538 (2003).
Wierinckx, A. et al. A diagnostic marker set for invasion, proliferation, and aggressiveness of prolactin pituitary tumors. Endocr. Relat. Cancer 14, 887–900 (2007).
Trouillas, J. et al. A new prognostic clinicopathological classification of pituitary adenomas: a multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 126, 123–135 (2013).
Raverot, G., Jouanneau, E. & Trouillas, J. Clinicopathological classification and molecular markers of pituitary tumours for personalized therapeutic strategies. Eur. J. Endocrinol. 170, R121–R132 (2014).
Monsalves, E. et al. Growth patterns of pituitary adenomas and histopathological correlates. J. Clin. Endocrinol. Metab. 99, 1330–1338 (2014).
Salehi, F. et al. Biomarkers of pituitary neoplasms: a review (Part II). Neurosurgery 67, 1790–1798 (2010).
Sav, A., Rotondo, F., Syro, L. V., Scheithauer, B. W. & Kovacs, K. Biomarkers of pituitary neoplasms. Anticancer Res. 32, 4639–4654 (2012).
Salehi, F. et al. Ki-67 in pituitary neoplasms: a review (Part I). Neurosurgery 65, 429–437 (2009).
Chiloiro, S. et al. Radically resected pituitary adenomas: prognostic role of Ki 67 labeling index in a monocentric retrospective series and literature review. Pituitary http://dx.doi.org/10.1007/s11102-013-0500–0506.
Kovacs, K. The 2004 WHO classification of pituitary tumors: comments. Acta Neuropathol. 111, 62–63 (2006).
Turner, H. E. & Wass, J. A. Are markers of proliferation valuable in the histological assessment of pituitary tumours? Pituitary 1, 147–151 (1999).
Thapar, K., Scheithauer, B. W., Kovacs, K., Pernicone, P. J. & Laws, E. R. Jr. p53 expression in pituitary adenomas and carcinomas: correlation with invasiveness and tumor growth fractions. Neurosurgery 38, 765–770 (1996).
Kontogeorgos, G. Predictive markers of pituitary adenoma behavior. Neuroendocrinology 83, 179–188 (2006).
Gejman, R., Swearingen, B. & Hedley-Whyte, E. T. Role of Ki-67 proliferation index and p53 expression in predicting progression of pituitary adenomas. Hum. Pathol. 39, 758–766 (2006).
Trouillas, J. et al. Polysialylated neural cell adhesion molecules expressed in human pituitary tumors and related to extrasellar invasion. J. Neurosurg. 98, 1084–1093 (2003).
Jaffe, C. A. & Barkan, A. L. Acromegaly. Recognition and treatment. Drugs 47, 425–445 (1994).
LeRiche, V. K., Asa, S. L & Ezzat, S. Epidermal growth factor and its receptors (EGF-R) in human pituitary adenomas: EGF-R correlates with tumor aggressiveness. J. Clin. Endocrinol. Metab. 81, 656–662 (1996).
Mete, O. et al. The role of mediators of cell invasiveness, motility, and migration in the pathogenesis of silent corticotroph adenomas. Endocr. Pathol. 24, 191–198 (2013).
Asa, S. L. & Ezzat, S. Genetics and proteomics of pituitary tumors. Endocrine 28, 43–47 (2005).
Lloyd, R. V., Vidal, S., Horvath, E, Kovacs, K. & Scheithauer, B. Angiogenesis in normal and neoplastic pituitary tissues. Microsc. Res. Tech. 60, 244–250 (2003).
Jugenburg, M., Kovacs, K., Stefaneanu, L. & Scheithauer, B. W. Vasculature in non-tumorous hypophyses, pituitary adenomas, and carcinomas: a quantitative morphological study. Endocr. Pathol. 6, 115–124 (1995).
Turner, H. E. et al. Angiogenesis in pituitary adenomas—relationship to endocrine function, treatment and outcome. J. Endocrinol. 165, 475–481 (2000).
Vidal, S. et al. Microvessel density in pituitary adenomas and carcinomas. Virchows Arch. 438, 595–602 (2001).
Di Ieva, A. et al. Euclidean and fractal geometry of microvascular networks in normal and neoplastic pituitary tissue. Neurosurg. Rev. 31, 271–281 (2008).
Di Ieva, A. et al. Fractal dimension as a quantitator of the microvasculature of normal and adenomatous pituitary tissue. J. Anat. 211, 673–680 (2007).
Di Ieva, A. et al. Microvascular morphometrics of the hypohysis and pituitary tumors: from bench to operating theatre. Microvasc. Res. 89, 7–14 (2013).
Zachary, I. VEGF signalling: integration and multi-tasking in endothelial cell biology. Biochem. Soc. Trans. 31, 1171–1177 (2003).
Carmeliet, P. & Jain, R. K. Molecular mechanisms and clinical applications of angiogenesis. Nature 473, 298–307 (2011).
Sánchez-Ortiga, R. et al. Over-expression of vascular endothelial growth factor in pituitary adenomas is associated with extrasellar growth and recurrence. Pituitary 16, 370–377 (2013).
Pan, L. X., Chen, Z. P., Liu, Y. S. & Zhao, J. H. Magnetic resonance imaging and biological markers in pituitary adenomas with invasion of the cavernous sinus space. J. Neurooncol. 74, 71–76 (2005).
Yarman, S. et al. Expression of Ki-67, p53 and vascular endothelial growth factor (VEGF) concomitantly in growth hormone-secreting pituitary adenomas: which one has a role in tumor behavior? Neuro. Endocrinol. Lett. 31, 823–828 (2010).
Bisht, S., Feldmann, G. & Brossart, P. Pharmacokinetics and pharmacodynamics of sunitinib for the treatment of advanced pancreatic neuroendocrine tumors. Expert Opin. Drug Metab. Toxicol. 9, 777–788 (2013).
Barroso-Sousa, R. et al. Complete resolution of hypercortisolism with sorafenib in a patient with advanced medullary thyroid carcinoma and ectopic ACTH syndrome. Thyroid http://dx.doi.org/10.1089/thy.2013.0571.
Jia, W. et al. Vascular endothelial growth inhibitor (VEGI) is an independent indicator for invasion in human pituitary adenomas. Anticancer Res. 33, 3815–3822 (2013).
Kaur, B. et al. Hypoxia and the hypoxia-factor pathway in glioma growth and angiogenesis. Neuro. Oncol. 7, 134–153 (2005).
Scherpereel, A. et al. Overexpression of endocan induces tumor formation. Cancer Res. 63, 6084–6089 (2003).
Maurage, C. A. et al. Endocan expression and localization in human glioblastomas. J. Neuropathol. Exp. Neurol. 68, 633–641 (2009).
Sarrazin, S. et al. Endocan or endothelial cell specific molecule-1 (ESM-1): a potential novel endothelial cell marker and a new target for cancer therapy. Biochim. Biophys. Acta 1765, 25–37 (2006).
Cornelius, A. et al. Endothelial expression of endocan is strongly associated with tumor progression in pituitary adenoma. Brain Pathol. 22, 757–764 (2012).
McCabe, C. J. et al. Vascular endothelial growth factor, its receptor KDR/FLK-1, and pituitary transforming gene in pituitary tumors. J. Clin. Endocrinol. Metab. 87, 4238–4244 (2002).
Minematsu, T. et al. PTTG overexpression is correlated with angiogenesis in human pituitary adenomas. Endocr. Pathol. 17, 143–153 (2006).
Chesnokova, V. & Melmed, S. Pituitary tumour-transforming gene (PTTG) and pituitary senescence. Horm. Res. 71 (Suppl. 2), 82–87 (2009).
Chesnokova, V. & Melmed, S. Pituitary senescence: the evolving role of PTTG. Mol. Cell Endocrinol. 326, 55–59 (2010).
Karga, H. J., Alexander, J. M., Hedley-Whyte, E. T., Klibanski, A. & Jameson, J. L. Ras mutations in human pituitary tumors. J. Clin. Endocr. Metab. 74, 914–919 (1992).
Cai, W. Y. et al. Ras mutations in human prolactinomas and pituitary carcinomas. J. Clin. Endocrinol. Metab. 78, 89–93 (1994).
Pei, L., Melmed, S., Scheithauer, B., Kovacs, K. & Prager, D. H-ras mutations in human pituitary carcinoma metastases. J. Clin. Endocrinol. Metab. 78, 842–846 (1994).
Rickert, C. H. et al. Increased chromosomal imbalances in recurrent pituitary adenomas. Acta Neuropathol. 102, 615–620 (2001).
Pack, S. D. et al. Common genetic changes in hereditary and sporadic pituitary adenomas detected by comparative genomic hybridization. Genes Chromosomes Cancer 43, 72–82 (2005).
Raverot, G. et al. Prognostic factors in prolactin pituitary tumors: clinical, histological, and molecular data from a series of 94 patients with a long postoperative follow-up. J. Clin. Endocrinol. Metab. 95, 1708–1716 (2010).
Wierinckx, A. et al. Integrated genomic profiling identifies loss of chromosome 11p impacting transcriptomic activity in aggressive pituitary PRL tumors. Brain Pathol. 21, 533–543 (2011).
Zemmoura, I. et al. Aggressive and malignant prolactin pituitary tumors: pathological diagnosis and patient management. Pituitary 16, 515–522 (2013).
Thapar, K. et al. Overexpression of the growth-hormone-releasing hormone gene in acromegaly-associated pituitary tumors. An event associated with neoplastic progression and aggressive behavior. Am. J. Pathol. 151, 769–784 (1997).
Gadelha, M. R., Trivellin, G., Hernandez Ramirez, L. C. & Korbonits, M. Genetics of pituitary adenomas. Front. Horm. Res. 41, 111–140 (2013).
Trouillas, J. et al. Pituitary tumors and hyperplasia in multiple endocrine neoplasia type 1 syndrome (MEN1): a case–control study in a series of 77 patients versus 2509 non-MEN1 patients. Am. J. Surg. Pathol. 32, 534–543 (2008).
Syro, L. V. et al. Pituitary tumors in patients with MEN1 syndrome. Clinics 67 (Suppl. 1), 43–48 (2012).
Toledo, S. P., Lourenco, D. M. Jr & Toledo, R. A. A differential diagnosis of inherited endocrine tumors and their tumor counterparts. Clinics (Sao Paulo) 68, 1039–1056 (2013).
Beckers, A., Aaltonen, L. A., Daly, A. F. & Karhu, A. Familial isolated pituitary adenomas (FIPA) and the pituitary adenoma predisposition due to mutations in the aryl hydrocarbon receptor interacting protein (AIP) gene. Endocr. Rev. 34, 239–277 (2013).
Pivonello, R. et al. Dopamine receptor expression and function in human normal adrenal gland and adrenal tumors. J. Clin. Endocrinol. Metab. 89, 4493–4502 (2004).
Gagliano, T. et al. Cabergoline reduces cell viability in non functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Pituitary 16, 91–100 (2013).
Molitch, M. E. Management of medically refractory prolactinoma. J. Neurooncol. http://dx.doi.org/10.1007/s11060-013-1270–1278.
Gillam, M. P., Molitch, M. P., Lombardi, G. & Colao, A. Advances in the treatment of prolactinomas. Endocr. Rev. 27, 485–534 (2006).
Oh, M. C. & Aghi, M. K. Dopamine agonist-resistant prolactinomas. J. Neurosurg. 114, 1369–1379 (2011).
Melmed, S. Acromegaly pathogenesis and treatment. J. Clin. Invest. 119, 3189–3202 (2009).
Kreutzer, J., Vance, M. L., Lopes, M. B. & Laws, E. R. Jr. Surgical management of GH-secreting pituitary adenomas: an outcome study using modern remission criteria. J. Clin. Endocrinol. Metab. 86, 4072–4077 (2001).
Wilson, T. J., McKean, E. L., Barkan, A. L., Chandler, W. F. & Sullivan, S. E. Repeat endoscopic transsphenoidal surgery for acromegaly: remission and complications. Pituitary 16, 459–464 (2013).
Melmed, S. et al. Acromegaly Consensus Group. Guidelines for acromegaly management: an update. J. Clin. Endocrinol. Metab. 94, 1509–1517 (2009).
Gola, M., Bonadonna, S., Mazziotti, G., Amato, G. & Giustina, A. Resistance to somatostatin analogs in acromegaly: an evolving concept? J. Endocrinol. Invest. 29, 86–93 (2006).
Colao, A., Auriemma, R. S., Lombardi, G. & Pivonello, R. Resistance to somatostatin analogs in acromegaly. Endocr. Rev. 32, 247–271 (2011).
Fougner, S. L., Casar-Borota, O., Heck, A., Berg, J. P. & Bollerslev, J. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin. Endocrinol. 76, 96–102 (2012).
Kato, M. et al. Differential expression of genes related to drug responsiveness between sparsely and densely granulated somatotroph adenomas. Endocr. J. 59, 221–228 (2012).
Kopchick, J. J., Parkinson, C., Stevens, E. C. & Trainer, P. J. Growth hormone receptor antagonists: discovery, development and use in patients with acromegaly. Endocr. Rev. 23, 623–646 (2002).
Parkinson, C. & Trainer, D. J. The place of pegvisomant in the management of acromegaly. Endocrinologist 13, 408–416 (2003).
Paisley, A. N. & Drake, W. H. Treatment of pituitary tumors. Pegvisomant. Endocrine 28, 111–114 (2005).
Kovacs, K. & Horvath, E. Effects of medical therapy on pituitary tumors. Ultrastruct. Pathol. 29, 163–167 (2005).
Drake, W. M., Berney, D. M., Kovacs, K. & Monson, J. P. Markers of cell proliferation in a GH-producing adenoma of a patient treated with pegvisomant. Eur. J. Endocrinol. 153, 203–205 (2005).
Horvath, E. & Kovacs, K. Pathology of acromegaly. Neuroendocrinology 83, 161–165 (2006).
Van der Lely et al. Long-term safety of pegvisomant in patients with acromegaly: comphrensive review of 1288 subjects in ACROSTUDY. J. Clin. Endocrinol. Metab. 97, 1589–1597 (2012).
Fleseriu, M. & Petersen, S. Medical management of Cushing's disease: what is the future? Pituitary 15, 330–341 (2012).
Colao, A., Boscaro, M., Ferone, D. & Casanueva, F. F. Managing Cushing's disease: the state of the art. Endocrine http://dx.doi.org/10.1007/s12020-013-0129–0122.
Pollock, B. E. & Young, W. F. Jr. Stereotactic radiosurgery for patients with ACTH-producing pituitary adenomas after prior adrenalectomy. Int. J. Radiat. Oncol. Biol. Phys. 54, 839–841 (2002).
Vik-Mo, E. O. et al. γ knife stereotactic radiosurgery of Nelson syndrome. Eur. J. Endocrinol. 160, 143–148 (2009).
Petit, J. H. et al. Proton stereotactic radiotherapy for persistent adrenocorticotropin-producing adenomas. J. Clin. Endocrinol. Metab. 93, 393–399 (2008).
Banasiak, M. J. & Malek, A. R. Nelson syndrome: comprehensive review of pathophysiology, diagnosis, and management. Neurosurg. Focus 23, E13 (2007).
Wagenmakers, M. A. et al. Endoscopic transsphenoidal pituitary surgery: a good and safe primary treatment option for Cushing's disease, even in case of macroadenomas or invasive adenomas. Eur. J. Endocrinol. 169, 329–337 (2013).
Pereira, A. M. & Biermasz, N. R. Treatment of nonfunctioning pituitary adenomas: what were the contributions of the last 10 years? A critical view. Ann. Endocrinol. (Paris) 73, 111–116 (2012).
Ding, D., Starke, R. M. & Sheehan, J. P. Treatment paradigms for pituitary adenomas: defining the roles of radiosurgery and radiation therapy. J. Neurooncol. http://dx.doi.org/10.1007/s11060-013-1262–1268.
Castinetti, F. et al. Outcome of γ knife radiosurgery in 82 patients with acromegaly: correlation with initial hypersecretion. Clin. Endocrinol. Metab. 90, 4483–4488 (2005).
Prasad, D. Clinical results of conformal radiotherapy and radiosurgery for pituitary adenoma. Neurosurg. Clin. N. Am. 17, 129–141 (2006).
Verma, J., McCutcheon, I. E., Waguespack, S. G. & Mahajan, A. Feasibility and outcome of re-irradiation in the treatment of multiply recurrent pituitary adenomas. Pituitary http://dx.doi.org/10.1007/s11102-013-0541-x.
Castinetti, F., Regis, J., Dufour, H. & Brue, T. Role of stereotactic radiosurgery in the management of pituitary adenomas. Nat. Rev. Endocrinol. 6, 214–223 (2010).
Minniti, G. et al. Risk of second brain tumor after conservative surgery and radiotherapy for pituitary adenoma: update after an additional 10 years. J. Clin. Endocrinol. Metab. 90, 800–804 (2005).
MacLean, J., Aldridge, M., Bomanji, J., Short, S. & Fersht, N. Peptide receptor radionuclide therapy for aggressive atypical pituitary adenoma/carcinoma: variable clinical response in preliminary evaluation. Pituitary http://dx.doi.org/10.1007/s11102-013-0540-y.
Mrugala, M. M. & Chamberlain, M. C. Mechanisms of disease: temozolomide and glioblastoma—look to the future. Nat. Clin. Pract Oncol. 5, 476–486 (2008).
Stupp, R., van den Bent, M. J. & Hegi, M. E. Optimal role of temozolomide in the treatment of malignant gliomas. Curr. Neurol. Neurosci. Rep. 5, 198–206 (2005).
Salehi. F. et al. O-6 methylguanine-DNA methyltransferase (MGMT) immunohistochemical expression in pituitary cortocotroph adenomas. Neurosurgery 70, 491–496 (2012).
Kovacs, K. et al. MGMT immunoexpression predicts responsiveness of pituitary tumors to temozolomide therapy. Acta Neuropathol. 115, 261–262 (2008).
Ortiz, L. D. et al. Temozolomide in aggressive pituitary adenomas and carcinomas. Clinics (Sao Paulo) 67 (Suppl. 1), 119–123 (2012).
Syro, L. V. et al. Antitumour effects of temozolomide in a man with a large, invasive prolactin-producing pituitary neoplasm. Clin. Endocrinol. (Oxf.) 65, 552–553 (2006).
Kovacs, K. et al. Temozolomide therapy in a man with an aggressive prolactin-secreting pituitary neoplasm: morphological findings. Hum. Pathol. 38, 185–189 (2007).
Syro, L. V. et al. Treatment of pituitary neoplasms with temozolomide: a review. Cancer 117, 454–462 (2011).
Moshkin, O. et al. Aggressive silent corticotroph adenoma progressing to pituitary carcinoma: the role of temozolomide therapy. Hormones (Athens) 10, 162–167 (2011).
Ortiz, L. D. et al. Anti-VEGF therapy in pituitary carcinoma. Pituitary 15, 445–449 (2012).
Bode, H. et al. SOM230 (pasireotide) and temozolomide achieve sustained control of tumour progression and ACTH secretion in pituitary carcinoma with widespread metastases. Exp. Clin. Endocrinol. Diabetes 118, 760–763 (2010).
Zacharia, B. E. et al. High response rates and prolonged survival in patients with corticotroph pituitary tumors and refractory Cushing's disease from capecitabine and temozolomide (CAPTEM): a case series. Neurosurgery 74, E447–E455 (2014).
Jouanneau, E. et al. New targeted therapies in pituitary carcinoma resistant to temozolomide. Pituitary 15, 37–43 (2012).
Fukuoka, H. et al. EGFR as a therapeutic target for human, canine, and mouse ACTH-secreting pituitary adenomas. J. Clin. Invest. 121, 4712–4721 (2011).
Fukuoka, H. et al. HER2/ErbB2 receptor signaling in rat and human prolactinoma cells: strategy for targeted prolactinoma therapy. Mol. Endocrinol. 25, 92–103 (2011).
Acknowledgements
The authors are grateful to the Jarislowsky and Lloyd Carr-Harris Foundations for their generous support.
Author information
Authors and Affiliations
Contributions
A.D.I., F.R. and L.V.S. researched data for the article. A.D.I. and K.K. made substantial contributions to discussions of the content. A.D.I. and L.V.S. wrote the article. A.D.I., F.R., L.V.S., M.D.C. and K.K. reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Di Ieva, A., Rotondo, F., Syro, L. et al. Aggressive pituitary adenomas—diagnosis and emerging treatments. Nat Rev Endocrinol 10, 423–435 (2014). https://doi.org/10.1038/nrendo.2014.64
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.64
This article is cited by
-
Neoplasms and tumor-like lesions of the sellar region: imaging findings with correlation to pathology and 2021 WHO classification
Neuroradiology (2023)
-
MRI and Trouillas’ grading system of pituitary tumors: the usefulness of T2 signal intensity volumetric values
Neuroradiology (2023)
-
Shape and texture analyses based on conventional MRI for the preoperative prediction of the aggressiveness of pituitary adenomas
European Radiology (2023)
-
Usefulness of a clinicopathological classification in predicting treatment-related outcomes and multimodal therapeutic approaches in pituitary adenoma patients: retrospective analysis on a Portuguese cohort of 129 patients from a tertiary pituitary center
Pituitary (2023)
-
Phosphorylation of β-catenin at Serine552 correlates with invasion and recurrence of non-functioning pituitary neuroendocrine tumours
Acta Neuropathologica Communications (2022)