Key Points
-
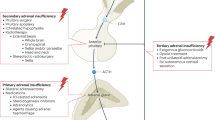
The health status in adults with congenital adrenal hyperplasia (CAH) is impaired, with an increased incidence of obesity, hypertension, osteoporosis and reduced quality of life and fertility
-
The poor health status of adults with CAH seems to be a result of their treatment; therefore, patient care needs to be improved and hormone replacement therapy optimized
-
Potent synthetic and long-acting glucocorticoids should only be used in patients with a clinical indication, and the dose should be maintained at the lowest level for the shortest time possible
-
Increasing the dose of glucocorticoids does not necessarily result in improved disease control, but will probably have adverse health consequences
-
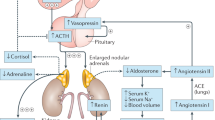
Hypertension is common, so mineralocorticoid replacement therapy should avoid suppressing plasma levels of renin below the normal range, and blood pressure should be monitored regularly in adults with CAH
Abstract
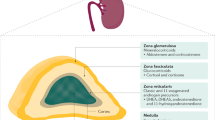
Congenital adrenal hyperplasia (CAH) is a genetic disorder caused by defective steroidogenesis that results in glucocorticoid deficiency; the most common underlying mutation is in the gene that encodes 21-hydroxylase. Life-saving glucocorticoid treatment was introduced in the 1950s, and the number of adult patients is now growing; however, no consensus has been reached on the management of CAH beyond childhood. Adult patients are prescribed a variety of glucocorticoids, including hydrocortisone, prednisone, prednisolone, dexamethasone and combinations of these drugs taken in either a circadian or reverse circadian regimen. Despite these personalized treatments, biochemical control of CAH is only achieved in approximately one-third of patients. Some patients have a poor health status, with an increased incidence of obesity and osteoporosis, and impaired fertility and quality of life. The majority of poor health outcomes seem to relate to inadequate treatment rather than the genotype of the patient. Patients receiving high doses of glucocorticoids and the more potent synthetic long-acting glucocorticoids are at an increased risk of obesity, insulin resistance and a reduced quality of life. Further research is required to optimize the treatment of adult patients with CAH and improve health outcomes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Pang, S. Y. et al. Worldwide experience in newborn screening for classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Pediatrics 81, 866–874 (1988).
White, P. C. & Speiser, P. W. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Endocr. Rev. 21, 245–291 (2000).
Miller, W. L. & Auchus, R. J. The molecular biology, biochemistry, and physiology of human steroidogenesis and its disorders. Endocr. Rev. 32, 81–151 (2011).
Speiser, P. W. et al. Congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 95, 4133–4160 (2010).
Merke, D. P. & Bornstein, S. R. Congenital adrenal hyperplasia. Lancet 365, 2125–2136 (2005).
Clayton, P. E. et al. Consensus statement on 21-hydroxylase deficiency from the European Society for Paediatric Endocrinology and the Lawson Wilkins Pediatric Endocrine Society. Horm. Res. 58, 188–195 (2002).
Arlt, W. et al. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. J. Clin. Endocrinol. Metab. 95, 5110–5121 (2010).
Reisch, N., Arlt, W. & Krone, N. Health problems in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Horm. Res. Paediatr. 76, 73–85 (2011).
Falhammar, H. & Thoren, M. Clinical outcomes in the management of congenital adrenal hyperplasia. Endocrine 41, 355–373 (2012).
Gidlof, S. et al. One hundred years of congenital adrenal hyperplasia in Sweden: a retrospective, population-based cohort study. Lancet Diabetes Endocrinol. 1, 35–42 (2013).
Finkielstain, G. P. et al. Comprehensive genetic analysis of 182 unrelated families with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 96, E161–E172 (2011).
Krone, N., Dhir, V., Ivison, H. E. & Arlt, W. Congenital adrenal hyperplasia and P450 oxidoreductase deficiency. Clin. Endocrinol. 66, 162–172 (2007).
Koppens, P. F., Hoogenboezem, T. & Degenhart, H. J. Duplication of the CYP21A2 gene complicates mutation analysis of steroid 21-hydroxylase deficiency: characteristics of three unusual haplotypes. Hum. Genet. 111, 405–410 (2002).
Therrell, B. L. Jr. Results of screening 1.9 million Texas newborns for 21-hydroxylase-deficient congenital adrenal hyperplasia. Pediatrics 101, 583–590 (1998).
Auchus, R. J. & Arlt, W. Approach to the patient: the adult with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 98, 2645–2655 (2013).
Speiser, P. W. et al. Disease expression and molecular genotype in congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Invest. 90, 584–595 (1992).
Wedell, A., Thilen, A., Ritzen, E. M., Stengler, B. & Luthman, H. Mutational spectrum of the steroid 21-hydroxylase gene in Sweden: implications for genetic diagnosis and association with disease manifestation. J. Clin. Endocrinol. Metab. 78, 1145–1152 (1994).
Krone, N. & Arlt, W. Genetics of congenital adrenal hyperplasia. Best Pract. Res. Clin. Endocrinol. Metab. 23, 181–192 (2009).
Charmandari, E. et al. Adrenomedullary function may predict phenotype and genotype in classic 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 87, 3031–3037 (2002).
Nimkarn, S. & New, M. I. Congenital adrenal hyperplasia due to 21-hydroxylase deficiency: A paradigm for prenatal diagnosis and treatment. Ann. NY Acad. Sci. 1192, 5–11 (2010).
Fitness, J. et al. Genotyping of CYP21, linked chromosome 6p markers, and a sex-specific gene in neonatal screening for congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 84, 960–966 (1999).
Krone, N., Braun, A., Roscher, A. A., Knorr, D. & Schwarz, H. P. Predicting phenotype in steroid 21-hydroxylase deficiency? Comprehensive genotyping in 155 unrelated, well defined patients from southern Germany. J. Clin. Endocrinol. Metab. 85, 1059–1065 (2000).
New, M. I. et al. Genotype–phenotype correlation in 1,507 families with congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Proc. Natl Acad. Sci. USA 110, 2611–2616 (2013).
Haider, S. et al. Structure-phenotype correlations of human CYP21A2 mutations in congenital adrenal hyperplasia. Proc. Natl Acad. Sci. USA 110, 2605–2610 (2013).
Nordenskjold, A. et al. Type of mutation and surgical procedure affect long-term quality of life for women with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 93, 380–386 (2008).
Frisen, L. et al. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. J. Clin. Endocrinol. Metab. 94, 3432–3439 (2009).
Krone, N. et al. Genotype-phenotype correlation in 153 adult patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency: analysis of the United Kingdom Congenital Adrenal Hyperplasia Adult Study Executive (CaHASE) cohort. J. Clin. Endocrinol. Metab. 98, E346–E354 (2013).
Speiser, P. W. et al. A summary of the Endocrine Society Clinical Practice Guidelines on congenital adrenal hyperplasia due to steroid 21-hydroxylase deficiency. Int. J. Pediatr. Endocrinol. 2010, 494173 (2010).
Debono, M. et al. Modified-release hydrocortisone to provide circadian cortisol profiles. J. Clin. Endocrinol. Metab. 94, 1548–1554 (2009).
Plat, L. et al. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J. Clin. Endocrinol. Metab. 84, 3082–3092 (1999).
Elbelt, U., Hahner, S. & Allolio, B. Altered insulin requirement in patients with type 1 diabetes and primary adrenal insufficiency receiving standard glucocorticoid replacement therapy. Eur. J. Endocrinol. 160, 919–924 (2009).
Spiegel, K., Leproult, R. & Van Cauter, E. Impact of sleep debt on metabolic and endocrine function. Lancet 354, 1435–1439 (1999).
Merke, D. P. Approach to the adult with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 93, 653–660 (2008).
Walker, B. R. Glucocorticoids and cardiovascular disease. Eur. J. Endocrinol. 157, 545–559 (2007).
Finkielstain, G. P. et al. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 97, 4429–4438 (2012).
Han, T. S. et al. Glucocorticoid treatment regimen and health outcomes in adults with congenital adrenal hyperplasia. Clin. Endocrinol. 78, 197–203 (2013).
Lin-Su, K. & New, M. I. Effects of adrenal steroids on the bone metabolism of children with congenital adrenal hyperplasia. Ann. NY Acad. Sci. 1117, 345–351 (2007).
German, A. et al. Control of childhood congenital adrenal hyperplasia and sleep activity and quality with morning or evening glucocorticoid therapy. J. Clin. Endocrinol. Metab. 93, 4707–4710 (2008).
Bryan, S. M., Honour, J. W. & Hindmarsh, P. C. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. J. Clin. Endocrinol. Metab. 94, 3477–3480 (2009).
Merza, Z. et al. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clin. Endocrinol. 65, 45–50 (2006).
Newell-Price, J. et al. Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers. Clin. Endocrinol. 68, 130–135 (2008).
Verma, S. et al. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin. Endocrinol. 72, 441–447 (2010).
Whitaker, M. et al. An oral multi-particulate, modified release, hydrocortisone replacement therapy that provides physiological cortisol exposure. Clin. Endocrinol. http://dx.doi.org/10.1111/cen.12316.
Auchus, R. J. et al. Marked androgen reduction in adult women with classic 21-hydroxylase deficiency (21OHD) treated with abiraterone acetate (AA) added to physiologic hydrocortisone (HC) and fludrocortisone (FC) [abstract LB-OR-1]. Presented at Endo2013.
Roche, E. F., Charmandari, E., Dattani, M. T. & Hindmarsh, P. C. Blood pressure in children and adolescents with congenital adrenal hyperplasia (21-hydroxylase deficiency): a preliminary report. Clin. Endocrinol. 58, 589–596 (2003).
Volkl, T. M., Simm, D., Dotsch, J., Rascher, W. & Dorr, H. G. Altered 24-hour blood pressure profiles in children and adolescents with classical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 91, 4888–4895 (2006).
Speiser, P. W. & White, P. C. Congenital adrenal hyperplasia. N. Engl. J. Med. 349, 776–788 (2003).
Sippell, W. G., Dorr, H. G., Bidlingmaier, F. & Knorr, D. Plasma levels of aldosterone, corticosterone, 11-deoxycorticosterone, progesterone, 17-hydroxyprogesterone, cortisol, and cortisone during infancy and childhood. Pediatr. Res. 14, 39–46 (1980).
Bauer, J. H. Age-related changes in the renin-aldosterone system. Physiological effects and clinical implications. Drugs Aging 3, 238–245 (1993).
Ogilvie, C. M., Rumsby, G., Kurzawinski, T. & Conway, G. S. Outcome of bilateral adrenalectomy in congenital adrenal hyperplasia: one unit's experience. Eur. J. Endocrinol. 154, 405–408 (2006).
Oelkers, W., Diederich, S. & Bahr, V. Diagnosis and therapy surveillance in Addison's disease: rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J. Clin. Endocrinol. Metab. 75, 259–264 (1992).
Young, M. C., Robinson, J. A., Read, G. F., Riad-Fahmy, D. & Hughes, I. A. 170H-progesterone rhythms in congenital adrenal hyperplasia. Arch. Dis. Child. 63, 617–623 (1988).
Sarafoglou, K. et al. Comparison of multiple steroid concentrations in serum and dried blood spots throughout the day of patients with congenital adrenal hyperplasia. Horm. Res. Pediatr. 75, 19–25 (2011).
Gastaud, F. et al. Impaired sexual and reproductive outcomes in women with classical forms of congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 92, 1391–1396 (2007).
Bidet, M. et al. Fertility in women with nonclassical congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 95, 1182–1190 (2010).
Casteras, A., De Silva, P., Rumsby, G. & Conway, G. S. Reassessing fecundity in women with classical congenital adrenal hyperplasia (CAH): normal pregnancy rate but reduced fertility rate. Clin. Endocrinol. 70, 833–837 (2009).
Mulaikal, R. M., Migeon, C. J. & Rock, J. A. Fertility rates in female patients with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. N. Engl. J. Med. 316, 178–182 (1987).
Hague, W. M. et al. The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin. Endocrinol. 33, 501–510 (1990).
Krege, S., Walz, K. H., Hauffa, B. P., Korner, I. & Rubben, H. Long-term follow-up of female patients with congenital adrenal hyperplasia from 21-hydroxylase deficiency, with special emphasis on the results of vaginoplasty. BJU Int. 86, 253–258 (2000).
Stikkelbroeck, N. M. et al. High prevalence of testicular adrenal rest tumors, impaired spermatogenesis, and Leydig cell failure in adolescent and adult males with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 86, 5721–5728 (2001).
Cabrera, M. S., Vogiatzi, M. G. & New, M. I. Long term outcome in adult males with classic congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 86, 3070–3078 (2001).
Claahsen-van der Grinten, H. L., Otten, B. J., Hermus, A. R., Sweep, F. C. & Hulsbergen-van de Kaa, C. A. Testicular adrenal rest tumors in patients with congenital adrenal hyperplasia can cause severe testicular damage. Fertil. Steril. 89, 597–601 (2008).
Avila, N. A., Premkumar, A. & Merke, D. P. Testicular adrenal rest tissue in congenital adrenal hyperplasia: comparison of MR imaging and sonographic findings. AJR Am. J. Roentgenol. 172, 1003–1006 (1999).
Russo, G., Paesano, P., Taccagni, G., Del Maschio, A. & Chiumello, G. Ovarian adrenal-like tissue in congenital adrenal hyperplasia. N. Engl. J. Med. 339, 853–854 (1998).
Martinez-Aguayo, A. et al. Testicular adrenal rest tumors and Leydig and Sertoli cell function in boys with classical congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 92, 4583–4589 (2007).
Reisch, N. et al. Testicular adrenal rest tumors develop independently of long-term disease control: A longitudinal analysis of 50 adult men with congenital adrenal hyperplasia due to classic 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 98, E1820–E1826 (2013).
Falhammar, H. et al. Fertility, sexuality and testicular adrenal rest tumors in adult males with congenital adrenal hyperplasia. Eur. J. Endocrinol. 166, 441–449 (2012).
Claahsen-van der Grinten, H. L., Otten, B. J., Sweep, F. C. & Hermus, A. R. Repeated successful induction of fertility after replacing hydrocortisone with dexamethasone in a patient with congenital adrenal hyperplasia and testicular adrenal rest tumors. Fertil. Steril. 88, 705.e5–8 (2007).
Bachelot, A., Chakthoura, Z., Rouxel, A., Dulon, J. & Touraine, P. Classical forms of congenital adrenal hyperplasia due to 21-hydroxylase deficiency in adults. Horm. Res. 69, 203–211 (2008).
Sugino, Y. et al. Genotyping of congenital adrenal hyperplasia due to 21-hydroxylase deficiency presenting as male infertility: case report and literature review. J. Assist. Reprod. Genet. 23, 377–380 (2006).
Scaroni, C. et al. Unilateral adrenal tumor, erectile dysfunction and infertility in a patient with 21-hydroxylase deficiency: effects of glucocorticoid treatment and surgery. Exp. Clin. Endocrinol. Diabetes 111, 41–43 (2003).
Tiosano, D. et al. Ovarian adrenal rest tumor in a congenital adrenal hyperplasia patient with adrenocorticotropin hypersecretion following adrenalectomy. Horm. Res. Paediatr. 74, 223–228 (2010).
Crocker, M. K. et al. Use of PET/CT with cosyntropin stimulation to identify and localize adrenal rest tissue following adrenalectomy in a woman with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 97, E2084–E2089 (2012).
McGeoch, S. C., Olson, S., Krukowski, Z. H. & Bevan, J. S. Giant bilateral myelolipomas in a man with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 97, 343–344 (2012).
Miller, W. L. Congenital adrenal hyperplasia in the adult patient. Adv. Intern. Med. 44, 155–173 (1999).
Lebbe, M. & Arlt, W. What is the best diagnostic and therapeutic management strategy for an Addison patient during pregnancy? Clin. Endocrinol. 78, 497–502 (2013).
Hirvikoski, T. et al. Cognitive functions in children at risk for congenital adrenal hyperplasia treated prenatally with dexamethasone. J. Clin. Endocrinol. Metab. 92, 542–548 (2007).
Altarescu, G. et al. Preimplantation genetic diagnosis (PGD)—prevention of the birth of children affected with endocrine diseases. J. Pediatr. Endocrinol. Metab. 24, 543–548 (2011).
Jaaskelainen, J. & Voutilainen, R. Long-term outcome of classical 21-hydroxylase deficiency: diagnosis, complications and quality of life. Acta Paediatr. 89, 183–187 (2000).
Reisch, N. et al. Quality of life is less impaired in adults with congenital adrenal hyperplasia because of 21-hydroxylase deficiency than in patients with primary adrenal insufficiency. Clin. Endocrinol. 74, 166–173 (2011).
Kuhnle, U. & Bullinger, M. Outcome of congenital adrenal hyperplasia. Pediatr. Surg. Int. 12, 511–515 (1997).
Johannsen, T. H. et al. Impaired cognitive function in women with congenital adrenal hyperplasia. J. Clin. Endocrinol. Metab. 91, 1376–1381 (2006).
Nermoen, I., Husebye, E. S., Svartberg, J. & Lovas, K. Subjective health status in men and women with congenital adrenal hyperplasia: a population-based survey in Norway. Eur. J. Endocrinol. 163, 453–459 (2010).
Han, T. S. et al. Quality of life in adults with congenital adrenal hyperplasia relates to glucocorticoid treatment, adiposity and insulin resistance: United Kingdom Congenital adrenal Hyperplasia Adult Study Executive (CaHASE). Eur. J. Endocrinol. 168, 887–893 (2013).
Meyer-Bahlburg, H. F., Dolezal, C., Baker, S. W., Ehrhardt, A. A. & New, M. I. Gender development in women with congenital adrenal hyperplasia as a function of disorder severity. Arch. Sex Behav. 35, 667–684 (2006).
Falhammar, H. et al. Metabolic profile and body composition in adult women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. J. Clin. Endocrinol. Metab. 92, 110–116 (2007).
Falhammar, H., Filipsson Nystrom, H., Wedell, A. & Thoren, M. Cardiovascular risk, metabolic profile, and body composition in adult males with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Eur. J. Endocrinol. 164, 285–293 (2011).
Mooij, C. F., Kroese, J. M., Claahsen-van der Grinten, H. L., Tack, C. J., Hermus, A. R. Unfavourable trends in cardiovascular and metabolic risk in paediatric and adult patients with congenital adrenal hyperplasia? Clin. Endocrinol. 73, 137–146 (2010).
Acknowledgements
The authors are grateful to all of the co-investigators in the UK congenital adrenal hyperplasia adult study executive, which is supported by the Clinical Endocrinology Trust and the Society for Endocrinology. W. Arlt would like to acknowledge the support of the Medical Research Council UK (Program Grant G0900567) and the European Community (FP7 Collaborative Research Project DSD-Life). B. R. Walker would like to acknowledge the support of the British Heart Foundation.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of the manuscript.
Corresponding author
Ethics declarations
Competing interests
R. J. Ross is a founding director and equity holder in Diurnal Ltd and W. Arlt is a consultant to Diurnal Ltd. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Han, T., Walker, B., Arlt, W. et al. Treatment and health outcomes in adults with congenital adrenal hyperplasia. Nat Rev Endocrinol 10, 115–124 (2014). https://doi.org/10.1038/nrendo.2013.239
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.239
This article is cited by
-
Chronotherapy based on modified-release hydrocortisone to restore the physiological cortisol diurnal rhythm
Drug Delivery and Translational Research (2023)
-
Long-term cardiometabolic morbidity in young adults with classic 21-hydroxylase deficiency congenital adrenal hyperplasia
Endocrine (2023)
-
The adrenal steroid profile in adolescent depression: a valuable bio-readout?
Translational Psychiatry (2022)
-
Adrenal hypofunction associated with ashwagandha (Withania somnifera) supplementation: a case report
Toxicology and Environmental Health Sciences (2022)
-
11-Oxygenated androgens in health and disease
Nature Reviews Endocrinology (2020)