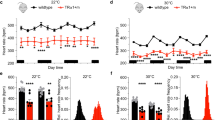
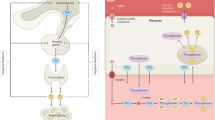
Key Points
-
The main isoforms of thyroid hormone receptors, THRα1, THRβ1 and THRβ2, are predominantly responsible for mediating thyroid hormone action, which is critical for normal development, growth and metabolism
-
Patients with mutations in either THRA or THRB have been described and have strikingly different clinical phenotypes known as resistance to thyroid hormone (RTH)α and RTHβ, respectively
-
Patients with RTHβ frequently present with elevated thyroid hormone levels, normal or elevated TSH levels and goitre, which suggests a critical role for THRB in negative-feedback regulation
-
Currently only seven patients with RTHα have been described; these individuals have near-normal levels of thyroid hormones and TSH but display hypothyroidism, delayed growth and constipation
-
Studies of mutations associated with RTH disorders using transgenic mouse models have provided novel insights into the divergent roles of THRA and THRB in physiology
Abstract
Thyroid hormone action is predominantly mediated by thyroid hormone receptors (THRs), which are encoded by the thyroid hormone receptor α (THRA) and thyroid hormone receptor β (THRB) genes. Patients with mutations in THRB present with resistance to thyroid hormone β (RTHβ), which is a disorder characterized by elevated levels of thyroid hormone, normal or elevated levels of TSH and goitre. Mechanistic insights about the contributions of THRβ to various processes, including colour vision, development of the cochlea and the cerebellum, and normal functioning of the adult liver and heart, have been obtained by either introducing human THRB mutations into mice or by deletion of the mouse Thrb gene. The introduction of the same mutations that mimic human THRβ alterations into the mouse Thra and Thrb genes resulted in distinct phenotypes, which suggests that THRA and THRB might have non-overlapping functions in human physiology. These studies also suggested that THRA mutations might not be lethal. Seven patients with mutations in THRα have since been described. These patients have RTHα and presented with major abnormalities in growth and gastrointestinal function. The hypothalamic–pituitary–thyroid axis in these individuals is minimally affected, which suggests that the central T3 feedback loop is not impaired in patients with RTHα, in stark contrast to patients with RTHβ.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Cheng, S. Y., Leonard, J. L. & Davis, P. J. Molecular aspects of thyroid hormone actions. Endocr. Rev. 31, 139–170 (2010).
Brent, G. A. Mechanisms of thyroid hormone action. J. Clin. Invest. 122, 3035–3043 (2012).
Chiamolera, M. I. & Wondisford, F. E. Minireview: Thyrotropin-releasing hormone and the thyroid hormone feedback mechanism. Endocrinology 150, 1091–1096 (2009).
Nikrodhanond, A. A. et al. Dominant role of thyrotropin-releasing hormone in the hypothalamic–pituitary–thyroid axis. J. Biol. Chem. 281, 5000–5007 (2006).
Gereben, B. et al. Cellular and molecular basis of deiodinase-regulated thyroid hormone signaling. Endocr. Rev. 29, 898–938 (2008).
Bianco, A. C. Minireview: cracking the metabolic code for thyroid hormone signaling. Endocrinology 152, 3306–3311 (2011).
Chiamolera, M. I. et al. Fundamentally distinct roles of thyroid hormone receptor isoforms in a thyrotroph cell line are due to differential DNA binding. Mol. Endocrinol. 26, 926–939 (2012).
Sap, J. et al. The c-erb-A protein is a high-affinity receptor for thyroid hormone. Nature 324, 635–640 (1986).
Oetting, A. & Yen, P. M. New insights into thyroid hormone action. Best Pract. Res. Clin. Endocrinol. Metab. 21, 193–208 (2007).
Refetoff, S. et al. Classification and proposed nomenclature for inherited defects of thyroid hormone action, cell transport, and metabolism. Thyroid 24, 407–409 (2014).
Shibusawa, N. et al. Thyroid hormone action in the absence of thyroid hormone receptor DNA-binding in vivo. J. Clin. Invest. 112, 588–597 (2003).
Ortiga-Carvalho, T. M. et al. Negative regulation by thyroid hormone receptor requires an intact coactivator-binding surface. J. Clin. Invest. 115, 2517–2523 (2005).
Kaneshige, M. et al. Mice with a targeted mutation in the thyroid hormone β receptor gene exhibit impaired growth and resistance to thyroid hormone. Proc. Natl Acad. Sci. USA 97, 13209–13214 (2000).
Ferrara, A. M. et al. Homozygous thyroid hormone receptor β-gene mutations in resistance to thyroid hormone: three new cases and review of the literature. J. Clin. Endocrinol. Metab. 97, 1328–1336 (2012).
Kaneshige, M. et al. A targeted dominant negative mutation of the thyroid hormone α1 receptor causes increased mortality, infertility, and dwarfism in mice. Proc. Natl Acad. Sci. USA 98, 15095–15100 (2001).
Bochukova, E. et al. A mutation in the thyroid hormone receptor α gene. N. Engl. J. Med. 366, 243–249 (2012).
Moran, C. et al. Resistance to thyroid hormone caused by a mutation in thyroid hormone receptor (TR)α1 and TRα2: clinical, biochemical, and genetic analyses of three related patients. Lancet Diabetes Endocrinol. http://dx.doi.org/10.1016/S2213-8587(14)70111-1.
Moran, C. et al. An adult female with resistance to thyroid hormone mediated by defective thyroid hormone receptor α. J. Clin. Endocrinol. Metab. 98, 4254–4261 (2013).
van Mullem, A. et al. Clinical phenotype and mutant TRα1. N. Engl. J. Med. 366, 1451–1453 (2012).
Refetoff, S. & Dumitrescu, A. M. Syndromes of reduced sensitivity to thyroid hormone: genetic defects in hormone receptors, cell transporters and deiodination. Best Pract. Res. Clin. Endocrinol. Metab. 21, 277–305 (2007).
Phan, T. Q., Jow, M. M. & Privalsky, M. L. DNA recognition by thyroid hormone and retinoic acid receptors: 3, 4, 5 rule modified. Mol. Cell Endocrinol. 319, 88–98 (2010).
Warnmark, A., Treuter, E., Wright, A. P. & Gustafsson, J. A. Activation functions 1 and 2 of nuclear receptors: molecular strategies for transcriptional activation. Mol. Endocrinol. 17, 1901–1909 (2003).
Figueira, A. C. et al. Analysis of agonist and antagonist effects on thyroid hormone receptor conformation by hydrogen/deuterium exchange. Mol. Endocrinol. 25, 15–31 (2011).
Chassande, O. et al. Identification of transcripts initiated from an internal promoter in the c-erbA α locus that encode inhibitors of retinoic acid receptor-α and triiodothyronine receptor activities. Mol. Endocrinol. 11, 1278–1290 (1997).
Gauthier, K. et al. Different functions for the thyroid hormone receptors TRα and TRβ in the control of thyroid hormone production and post-natal development. EMBO J. 18, 623–631 (1999).
Williams, G. R. Cloning and characterization of two novel thyroid hormone receptor β isoforms. Mol. Cell Biol. 20, 8329–8342 (2000).
Wallis, K. et al. The thyroid hormone receptor α1 protein is expressed in embryonic postmitotic neurons and persists in most adult neurons. Mol. Endocrinol. 24, 1904–1916 (2010).
Bradley, D. J., Towle, H. C. & Young, W. S. 3rd. Spatial and temporal expression of α- and β-thyroid hormone receptor mRNAs, including the β2-subtype, in the developing mammalian nervous system. J. Neurosci. 12, 2288–2302 (1992).
Bradley, D. J., Towle, H. C. & Young, W. S. 3rd. α and β thyroid hormone receptor (TR) gene expression during auditory neurogenesis: evidence for TR isoform-specific transcriptional regulation in vivo. Proc. Natl Acad. Sci. USA 91, 439–443 (1994).
Kilby, M. D. et al. Circulating thyroid hormone concentrations and placental thyroid hormone receptor expression in normal human pregnancy and pregnancy complicated by intrauterine growth restriction (IUGR). J. Clin. Endocrinol. Metab. 83, 2964–2971 (1998).
Feng, X., Jiang, Y., Meltzer, P. & Yen, P. M. Thyroid hormone regulation of hepatic genes in vivo detected by complementary DNA microarray. Mol. Endocrinol. 14, 947–955 (2000).
Flores-Morales, A. et al. Patterns of liver gene expression governed by TRβ. Mol. Endocrinol. 16, 1257–1268 (2002).
de Araujo, A. S., Martinez, L., de Paula Nicoluci, R., Skaf, M. S. & Polikarpov, I. Structural modeling of high-affinity thyroid receptor-ligand complexes. Eur. Biophys. J. 39, 1523–1536 (2010).
Valadares, N. F., Polikarpov, I. & Garratt, R. C. Ligand induced interaction of thyroid hormone receptor β with its coregulators. J. Steroid Biochem. Mol. Biol. 112, 205–212 (2008).
Weiss, R. E. et al. Dominant inheritance of resistance to thyroid hormone not linked to defects in the thyroid hormone receptor α or β genes may be due to a defective cofactor. J. Clin. Endocrinol. Metab. 81, 4196–4203 (1996).
Souza, P. C. et al. Helix 12 dynamics and thyroid hormone receptor activity: experimental and molecular dynamics studies of Ile280 mutants. J. Mol. Biol. 412, 882–893 (2011).
Alonso, M. et al. In vivo interaction of steroid receptor coactivator (SRC)-1 and the activation function-2 domain of the thyroid hormone receptor (TR) β in TRβ E457A knock-in and SRC-1 knockout mice. Endocrinology 150, 3927–3934 (2009).
Lavery, D. N. & McEwan, I. J. Structure and function of steroid receptor AF1 transactivation domains: induction of active conformations. Biochem. J. 391, 449–464 (2005).
Cohen, R. N. et al. The specificity of interactions between nuclear hormone receptors and corepressors is mediated by distinct amino acid sequences within the interacting domains. Mol. Endocrinol. 15, 1049–1061 (2001).
Hollenberg, A. N., Monden, T. & Wondisford, F. E. Ligand-independent and -dependent functions of thyroid hormone receptor isoforms depend upon their distinct amino termini. J. Biol. Chem. 270, 14274–14280 (1995).
Hashimoto, K. et al. Cross-talk between thyroid hormone receptor and liver X receptor regulatory pathways is revealed in a thyroid hormone resistance mouse model. J. Biol. Chem. 281, 295–302 (2006).
Liu, Y. Y. et al. A mutant thyroid hormone receptor α antagonizes peroxisome proliferator-activated receptor α signaling in vivo and impairs fatty acid oxidation. Endocrinology 148, 1206–1217 (2007).
Brent, G. A. et al. Capacity for cooperative binding of thyroid hormone (T3) receptor dimers defines wild type T3 response elements. Mol. Endocrinol. 6, 502–514 (1992).
Ayers, S. et al. Genome-wide binding patterns of thyroid hormone receptor β. PLoS One 9, e81186 (2014).
Chatonnet, F., Guyot, R., Benoit, G. & Flamant, F. Genome-wide analysis of thyroid hormone receptors shared and specific functions in neural cells. Proc. Natl Acad. Sci. USA 110, E766–E775 (2013).
Astapova, I. et al. The nuclear corepressor, NCoR, regulates thyroid hormone action in vivo. Proc. Natl Acad. Sci. USA 105, 19544–19549 (2008).
Feng, W. et al. Hormone-dependent coactivator binding to a hydrophobic cleft on nuclear receptors. Science 280, 1747–1749 (1998).
Darimont, B. D. et al. Structure and specificity of nuclear receptor-coactivator interactions. Genes Dev. 12, 3343–3356 (1998).
McKenna, N. J. et al. Nuclear receptor coactivators: multiple enzymes, multiple complexes, multiple functions. J. Steroid Biochem. Mol. Biol. 69, 3–12 (1999).
Shibusawa, N., Hollenberg, A. N. & Wondisford, F. E. Thyroid hormone receptor DNA binding is required for both positive and negative gene regulation. J. Biol. Chem. 278, 732–738 (2003).
Sasaki, S. et al. Ligand-induced recruitment of a histone deacetylase in the negative-feedback regulation of the thyrotropin β gene. EMBO J. 18, 5389–5398 (1999).
Santos, G. M. et al. Negative regulation of superoxide dismutase-1 promoter by thyroid hormone. Mol. Pharmacol. 70, 793–800 (2006).
Weiss, R. E. et al. Mice deficient in the steroid receptor co-activator 1 (SRC-1) are resistant to thyroid hormone. EMBO J. 18, 1900–1904 (1999).
Kamiya, Y. et al. Modulation by steroid receptor coactivator-1 of target-tissue responsiveness in resistance to thyroid hormone. Endocrinology 144, 4144–4153 (2003).
Costa-e-Sousa, R. H., Astapova, I., Ye, F., Wondisford, F. E. & Hollenberg, A. N. The thyroid axis is regulated by NCoR1 via its actions in the pituitary. Endocrinology 153, 5049–5057 (2012).
Aninye, I. O., Matsumoto, S., Sidhaye, A. R. & Wondisford, F. E. Circadian regulation of Tshb gene expression by Rev-Erbα (NR1D1) and nuclear corepressor 1 (NCOR1). J. Biol. Chem. 289, 17070–17077 (2014).
Treuter, E., Albrektsen, T., Johansson, L., Leers, J. & Gustafsson, J. A. A regulatory role for RIP140 in nuclear receptor activation. Mol. Endocrinol. 12, 864–881 (1998).
Ramadoss, P. et al. Novel mechanism of positive versus negative regulation by thyroid hormone receptor β1 (TRβ1) identified by genome-wide profiling of binding sites in mouse liver. J. Biol. Chem. 289, 1313–1328 (2014).
Abel, E. D., Ahima, R. S., Boers, M. E., Elmquist, J. K. & Wondisford, F. E. Critical role for thyroid hormone receptor β2 in the regulation of paraventricular thyrotropin-releasing hormone neurons. J. Clin. Invest. 107, 1017–1023 (2001).
Gauthier, K. et al. Genetic analysis reveals different functions for the products of the thyroid hormone receptor α locus. Mol. Cell Biol. 21, 4748–4760 (2001).
Macchia, P. E. et al. Increased sensitivity to thyroid hormone in mice with complete deficiency of thyroid hormone receptor α. Proc. Natl Acad. Sci. USA 98, 349–354 (2001).
Gothe, S. et al. Mice devoid of all known thyroid hormone receptors are viable but exhibit disorders of the pituitary-thyroid axis, growth, and bone maturation. Genes Dev. 13, 1329–1341 (1999).
Pazos-Moura, C. et al. Cardiac dysfunction caused by myocardium-specific expression of a mutant thyroid hormone receptor. Circ. Res. 86, 700–706 (2000).
Ono, S. et al. Homozygosity for a dominant negative thyroid hormone receptor gene responsible for generalized resistance to thyroid hormone. J. Clin. Endocrinol. Metab. 73, 990–994 (1991).
Usala, S. J. et al. A homozygous deletion in the c-erbA β thyroid hormone receptor gene in a patient with generalized thyroid hormone resistance: isolation and characterization of the mutant receptor. Mol. Endocrinol. 5, 327–335 (1991).
Takeda, K., Sakurai, A., DeGroot, L. J. & Refetoff, S. Recessive inheritance of thyroid hormone resistance caused by complete deletion of the protein-coding region of the thyroid hormone receptor-β gene. J. Clin. Endocrinol. Metab. 74, 49–55 (1992).
van Mullem, A. A. et al. Clinical phenotype of a new type of thyroid hormone resistance caused by a mutation of the TRα1 receptor: consequences of LT4 treatment. J. Clin. Endocrinol. Metab. 98, 3029–3038 (2013).
Forrest, D., Erway, L. C., Ng, L., Altschuler, R. & Curran, T. Thyroid hormone receptor β is essential for development of auditory function. Nat. Genet. 13, 354–357 (1996).
Forrest, D. & Vennstrom, B. Functions of thyroid hormone receptors in mice. Thyroid 10, 41–52 (2000).
Abel, E. D. et al. Divergent roles for thyroid hormone receptor β isoforms in the endocrine axis and auditory system. J. Clin. Invest. 104, 291–300 (1999).
Dettling, J. et al. Autonomous functions of murine thyroid hormone receptor TRα and TRβ in cochlear hair cells. Mol. Cell Endocrinol. 382, 26–37 (2013).
Portella, A. C. et al. Thyroid hormone receptor β mutation causes severe impairment of cerebellar development. Mol. Cell Neurosci. 44, 68–77 (2010).
Hashimoto, K. et al. An unliganded thyroid hormone receptor causes severe neurological dysfunction. Proc. Natl Acad. Sci. USA 98, 3998–4003 (2001).
Machado, D. S. et al. A thyroid hormone receptor mutation that dissociates thyroid hormone regulation of gene expression in vivo. Proc. Natl Acad. Sci. USA 106, 9441–9446 (2009).
Parrilla, R., Mixson, A. J., McPherson, J. A., McClaskey, J. H. & Weintraub, B. D. Characterization of seven novel mutations of the c-erbA β gene in unrelated kindreds with generalized thyroid hormone resistance. Evidence for two “hot spot” regions of the ligand binding domain. J. Clin. Invest. 88, 2123–2130 (1991).
Flynn, T. R. et al. A novel C-terminal domain in the thyroid hormone receptor selectively mediates thyroid hormone inhibition. J. Biol. Chem. 269, 32713–32716 (1994).
Faustino, L. C. et al. Liver glutathione S-transferase α expression is decreased by T3 in hypothyroidism but not in euthyroidism in mice. Exp. Physiol. 96, 790–800 (2011).
Cordas, E. A. et al. Thyroid hormone receptors control developmental maturation of the middle ear and the size of the ossicular bones. Endocrinology 153, 1548–1560 (2012).
Richter, C. P., Munscher, A., Machado, D. S., Wondisford, F. E. & Ortiga-Carvalho, T. M. Complete activation of thyroid hormone receptor β by T3 is essential for normal cochlear function and morphology in mice. Cell Physiol. Biochem. 28, 997–1008 (2011).
Pessoa, C. N. et al. Thyroid hormone action is required for normal cone opsin expression during mouse retinal development. Invest. Ophthalmol. Vis. Sci. 49, 2039–2045 (2008).
Ng, L. et al. A thyroid hormone receptor that is required for the development of green cone photoreceptors. Nat. Genet. 27, 94–98 (2001).
Roberts, M. R., Srinivas, M., Forrest, D., Morreale de Escobar, G. & Reh, T. A. Making the gradient: thyroid hormone regulates cone opsin expression in the developing mouse retina. Proc. Natl Acad. Sci. USA 103, 6218–6223 (2006).
Weiss, A. H., Kelly, J. P., Bisset, D. & Deeb, S. S. Reduced L- and M- and increased S-cone functions in an infant with thyroid hormone resistance due to mutations in the THRβ2 gene. Ophthalmic Genet. 33, 187–195 (2012).
Mangelsdorf, D. J. & Evans, R. M. The RXR heterodimers and orphan receptors. Cell 83, 841–850 (1995).
Hollenberg, A. N., Monden, T., Madura, J. P., Lee, K. & Wondisford, F. E. Function of nuclear co-repressor protein on thyroid hormone response elements is regulated by the receptor A/B domain. J. Biol. Chem. 271, 28516–28520 (1996).
Liu, Y., Xia, X., Fondell, J. D. & Yen, P. M. Thyroid hormone-regulated target genes have distinct patterns of coactivator recruitment and histone acetylation. Mol. Endocrinol. 20, 483–490 (2006).
Fozzatti, L. et al. Resistance to thyroid hormone is modulated in vivo by the nuclear receptor corepressor (NCOR1). Proc. Natl Acad. Sci. USA 108, 17462–17467 (2011).
Swanson, E. A. et al. Cardiac expression and function of thyroid hormone receptor β and its PV mutant. Endocrinology 144, 4820–4825 (2003).
Suarez, J., Scott, B. T., Suarez-Ramirez, J. A., Chavira, C. V. & Dillmann, W. H. Thyroid hormone inhibits ERK phosphorylation in pressure overload-induced hypertrophied mouse hearts through a receptor-mediated mechanism. Am. J. Physiol. Cell Physiol. 299, C1524–C1529 (2010).
Gloss, B. et al. Cardiac ion channel expression and contractile function in mice with deletion of thyroid hormone receptor α or β. Endocrinology 142, 544–550 (2001).
Gloss, B. et al. Altered cardiac phenotype in transgenic mice carrying the Δ337 threonine thyroid hormone receptor β mutant derived from the S family. Endocrinology 140, 897–902 (1999).
Ortiga-Carvalho, T. M. et al. Thyroid hormone resistance in the heart: role of the thyroid hormone receptor β isoform. Endocrinology 145, 1625–1633 (2004).
Safer, J. D. et al. Isoform variable action among thyroid hormone receptor mutants provides insight into pituitary resistance to thyroid hormone. Mol. Endocrinol. 11, 16–26 (1997).
Wagner, R. L. et al. A structural role for hormone in the thyroid hormone receptor. Nature 378, 690–697 (1995).
Nagaya, T. & Jameson, J. L. Thyroid hormone receptor dimerization is required for dominant negative inhibition by mutations that cause thyroid hormone resistance. J. Biol. Chem. 268, 15766–15771 (1993).
Yen, P. M., Wilcox, E. C., Hayashi, Y., Refetoff, S. & Chin, W. W. Studies on the repression of basal transcription (silencing) by artificial and natural human thyroid hormone receptor-β mutants. Endocrinology 136, 2845–2851 (1995).
Monden, T. et al. Leucine at codon 428 in the ninth heptad of thyroid hormone receptor β1 is necessary for interactions with the transcriptional cofactors and functions regardless of dimer formations. Thyroid 13, 427–435 (2003).
Fraichard, A. et al. The T3R α gene encoding a thyroid hormone receptor is essential for post-natal development and thyroid hormone production. EMBO J. 16, 4412–4420 (1997).
O'Shea, P. J. et al. A thyrotoxic skeletal phenotype of advanced bone formation in mice with resistance to thyroid hormone. Mol. Endocrinol. 17, 1410–1424 (2003).
O'Shea, P. J. et al. Contrasting skeletal phenotypes in mice with an identical mutation targeted to thyroid hormone receptor α1 or β. Mol. Endocrinol. 19, 3045–3059 (2005).
Quignodon, L., Vincent, S., Winter, H., Samarut, J. & Flamant, F. A point mutation in the activation function 2 domain of thyroid hormone receptor α1 expressed after CRE-mediated recombination partially recapitulates hypothyroidism. Mol. Endocrinol. 21, 2350–2360 (2007).
Fauquier, T. et al. Purkinje cells and Bergmann glia are primary targets of the TRα1 thyroid hormone receptor during mouse cerebellum postnatal development. Development 141, 166–175 (2014).
Adams, M. et al. Genetic analysis of 29 kindreds with generalized and pituitary resistance to thyroid hormone. Identification of thirteen novel mutations in the thyroid hormone receptor β gene. J. Clin. Invest. 94, 506–515 (1994).
Tinnikov, A. et al. Retardation of post-natal development caused by a negatively acting thyroid hormone receptor α1. EMBO J. 21, 5079–5087 (2002).
Refetoff, S., DeWind, L. T. & DeGroot L. J. Familial syndrome combining deaf-mutism, stuppled epiphyses, goiter and abnormally high PBI: possible target organ refractoriness to thyroid hormone. J. Clin. Endocrinol. Metab. 27, 279–294 (1967).
Liu, Y. Y., Schultz, J. J. & Brent, G. A. A thyroid hormone receptor α gene mutation (P398H) is associated with visceral adiposity and impaired catecholamine-stimulated lipolysis in mice. J. Biol. Chem. 278, 38913–38920 (2003).
Acknowledgements
T.M.O.-C.'s research is supported by Fundação Carlos Chagas Filho de Amparo a Pesquisa do Estado do Rio de Janeiro (FAPERJ, CNE 102.873/2012) and Conselho Nacional de Pesquisa e Desenvolvimento (CNPq, #303,734/2012-4) and the Bill and Melinda Gates Foundation. F.E.W.'s research is supported by National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant R01 DK49126 and the Johns Hopkins–University of Maryland Diabetes Research Center NIDDK grant P30DK79637.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to all aspects of this article.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Ortiga-Carvalho, T., Sidhaye, A. & Wondisford, F. Thyroid hormone receptors and resistance to thyroid hormone disorders. Nat Rev Endocrinol 10, 582–591 (2014). https://doi.org/10.1038/nrendo.2014.143
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2014.143
This article is cited by
-
Changing the structure of PFOA and PFOS: a chemical industry strategy or a solution to avoid thyroid-disrupting effects?
Journal of Endocrinological Investigation (2024)
-
Brain nuclear receptors and cardiovascular function
Cell & Bioscience (2023)
-
Overlaps Between CDS Regions of Protein-Coding Genes in the Human Genome: A Case Study on the NR1D1-THRA Gene Pair
Journal of Molecular Evolution (2023)
-
Thyroid Hormones and Skeletal Muscle Beyond Thermogenesis
Journal of Science in Sport and Exercise (2023)
-
Gene polymorphisms and thyroid hormone signaling: implication for the treatment of hypothyroidism
Endocrine (2023)