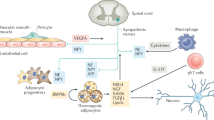
Key Points
-
The term browning describes the emergence of beige adipocytes in white adipose tissue—a reversible process that represents adaptation to increased thermogenic demand and exercise
-
Human brown adipose tissue is diverse and consists of both brown and beige adipocytes, in proportions that differ according to the fat depot's anatomical location and the age of the person
-
Beige adipocytes are generated by both de novo recruitment from progenitor cells and transdifferentiation from white adipocytes—independent processes that might coexist
-
Cellular energy sensing, in addition to sympathetic tone, are the driving forces that regulate the transcriptional networks controlling browning
-
Cold exposure and other metabolic challenges elicit complex hormonal responses that facilitate communication between tissues and prepare the body for adaptive thermogenesis
-
Brown adipose tissue is a critical regulator of metabolic health in mice; yet, whether induction of browning will be a promising avenue to treat metabolic disorders in humans remains unclear
Abstract
Accumulation of excess white adipose tissue (WAT) has deleterious consequences for metabolic health. The activation of brown adipose tissue (BAT), the primary organ for heat production, confers beneficial effects on adiposity, insulin resistance and hyperlipidaemia, at least in mice. As the amount of metabolically active BAT seems to be particularly low in patients with obesity or diabetes mellitus who require immediate therapy, new avenues are needed to increase the capacity for adaptive thermogenesis. In this light, we review the findings that BAT in human adults might consist of not only classic brown adipocytes but also inducible brown adipocytes (also called beige, brown-in-white, or brite adipocytes), which are phenotypically distinct from both white and brown adipocytes. Stimulating the development of beige adipocytes in WAT (so called 'browning') might reduce adverse effects of WAT and could help to improve metabolic health. This article focuses on the development and regulatory control of beige adipocytes at the transcriptional and hormonal levels. Emerging insights into the metabolic role of beige adipocytes are also discussed, along with the developments that can be expected from these promising targets for therapy of metabolic disease in the future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Zechner, R. et al. FAT SIGNALS—lipases and lipolysis in lipid metabolism and signalling. Cell Metab. 15, 279–291 (2012).
Gesta, S., Tseng, Y. H. & Kahn, C. R. Developmental origin of fat: tracking obesity to its source. Cell 131, 242–256 (2007).
Cannon, B. & Nedergaard, J. Brown adipose tissue: function and physiological significance. Physiol. Rev. 84, 277–359 (2004).
van Marken Lichtenbelt, W. D. et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 360, 1500–1508 (2009).
Virtanen, K. A. et al. Functional brown adipose tissue in healthy adults. N. Engl. J. Med. 360, 1518–1525 (2009).
Yoneshiro, T. et al. Brown adipose tissue, whole-body energy expenditure, and thermogenesis in healthy adult men. Obesity (Silver Spring) 19, 13–16 (2011).
Ouellet, V. et al. Outdoor temperature, age, sex, body mass index, and diabetic status determine the prevalence, mass, and glucose-uptake activity of 18F-FDG-detected BAT in humans. J. Clin. Endocrinol. Metab. 96, 192–199 (2011).
van der Lans, A. A. et al. Cold acclimation recruits human brown fat and increases nonshivering thermogenesis. J. Clin. Invest. 123, 3395–3403 (2013).
Yoneshiro, T. et al. Recruited brown adipose tissue as an antiobesity agent in humans. J. Clin. Invest. 123, 3404–3408 (2013).
Cinti, S. The adipose organ at a glance. Dis. Model. Mech. 5, 588–594 (2012).
Klingenspor, M., Herzig, S. & Pfeifer, A. Brown fat develops a brite future. Obes. Facts 5, 890–896 (2012).
Nedergaard, J., Bengtsson, T. & Cannon, B. Unexpected evidence for active brown adipose tissue in adult humans. Am. J. Physiol. Endocrinol. Metab. 293, E444–E452 (2007).
Cypess, A. M. et al. Identification and importance of brown adipose tissue in adult humans. N. Engl. J. Med. 360, 1509–1517 (2009).
Zingaretti, M. C. et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 23, 3113–3120 (2009).
Saito, M. et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: effects of cold exposure and adiposity. Diabetes 58, 1526–1531 (2009).
Pfannenberg, C. et al. Impact of age on the relationships of brown adipose tissue with sex and adiposity in humans. Diabetes 59, 1789–1793 (2010).
Lowell, B. B. et al. Development of obesity in transgenic mice after genetic ablation of brown adipose tissue. Nature 366, 740–742 (1993).
Enerbäck, S. et al. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature 387, 90–94 (1997).
Feldmann, H. M., Golozoubova, V., Cannon, B. & Nedergaard, J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metab. 9, 203–209 (2009).
Kopecky, J., Clarke, G., Enerbäck, S., Spiegelman, B. & Kozak, L. P. Expression of the mitochondrial uncoupling protein gene from the aP2 gene promoter prevents genetic obesity. J. Clin. Invest 96, 2914–2923 (1995).
Van Gaal, L. F., Mertens, I. L. & De Block, C. E. Mechanisms linking obesity with cardiovascular disease. Nature 444, 875–880 (2006).
Guilherme, A., Virbasius, J. V., Puri, V. & Czech, M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 367–377 (2008).
Bartelt, A. & Heeren, J. The holy grail of metabolic disease: brown adipose tissue. Curr. Opin. Lipidol. 23, 190–195 (2012).
Nedergaard, J. & Cannon, B. How brown is brown fat? It depends where you look. Nat. Med. 19, 540–541 (2013).
Timmons, J. A. et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc. Natl Acad. Sci. USA 104, 4401–4406 (2007).
Seale, P. et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature 454, 961–967 (2008).
Tran, K. V. et al. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 15, 222–229 (2012).
Lee, Y. H., Petkova, A. P., Mottillo, E. P. & Granneman, J. G. In vivo identification of bipotential adipocyte progenitors recruited by β3-adrenoceptor activation and high-fat feeding. Cell Metab. 15, 480–491 (2012).
Gupta, R. K. et al. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 15, 230–239 (2012).
Petrovic, N. et al. Chronic peroxisome proliferator-activated receptor gamma (PPARγ) activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classic brown adipocytes. J. Biol. Chem. 285, 7153–7164 (2010).
Klaus, S., Ely, M., Encke, D. & Heldmaier, G. Functional assessment of white and brown adipocyte development and energy metabolism in cell culture. Dissociation of terminal differentiation and thermogenesis in brown adipocytes. J. Cell Sci. 108, 3171–3180 (1995).
Schulz, T. J. et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc. Natl Acad. Sci. USA 108, 143–148 (2011).
Wu, J. et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cell 150, 366–376 (2012).
Himms-Hagen, J. et al. Multilocular fat cells in WAT of CL-316243-treated rats derive directly from white adipocytes. Am. J. Physiol. Cell Physiol. 279, C670–C681 (2000).
Barbatelli, G. et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am. J. Physiol. Endocrinol. Metab. 298, E1244–E1253 (2010).
Rosenwald, M., Perdikari, A., Rülicke, T. & Wolfrum, C. Bi-directional interconversion of brite and white adipocytes. Nat. Cell Biol. 15, 659–667 (2013).
Sharp, L. Z. et al. Human BAT possesses molecular signatures that resemble beige/brite cells. PLoS ONE 7, e49452 (2012).
Cypess, A. M. et al. Anatomical localization, gene expression profiling and functional characterization of adult human neck brown fat. Nat. Med. 19, 635–639 (2013).
Jespersen, N. Z. et al. A classical brown adipose tissue mRNA signature partly overlaps with brite in the supraclavicular region of adult humans. Cell Metab. 17, 798–805 (2013).
Lidell, M. E. et al. Evidence for two types of brown adipose tissue in humans. Nat. Med. 19, 631–634 (2013).
Walden, T. B., Hansen, I. R., Timmons, J. A., Cannon, B. & Nedergaard, J. Recruited vs. nonrecruited molecular signatures of brown, “brite, ” and white adipose tissues. Am. J. Physiol. Endocrinol. Metab. 302, E19–E31 (2012).
Farmer, S. R. Transcriptional control of adipocyte formation. Cell Metab. 4, 263–273 (2006).
Rosen, E. D. & MacDougald, O. A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 7, 885–896 (2006).
Linhart, H. G. et al. C/EBPα is required for differentiation of white, but not brown, adipose tissue. Proc. Natl Acad. Sci. USA 98, 12532–12537 (2001).
Gesta, S. et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc. Natl Acad. Sci. USA 103, 6676–6681 (2006).
Lee, K. Y. et al. Shox2 is a molecular determinant of depot-specific adipocyte function. Proc. Natl Acad. Sci. USA 110, 11409–11414 (2013).
Cederberg, A. et al. FOXC2 is a winged helix gene that counteracts obesity, hypertriglyceridemia, and diet-induced insulin resistance. Cell 106, 563–573 (2001).
Hansen, J. B. et al. Retinoblastoma protein functions as a molecular switch determining white versus brown adipocyte differentiation. Proc. Natl Acad. Sci. USA 101, 4112–4117 (2004).
Calo, E. et al. Rb regulates fate choice and lineage commitment in vivo. Nature 466, 1110–1114 (2010).
Scime, A. et al. Rb and p107 regulate preadipocyte differentiation into white versus brown fat through repression of PGC-1α. Cell Metab. 2, 283–295 (2005).
Tsukiyama-Kohara, K. et al. Adipose tissue reduction in mice lacking the translational inhibitor 4E-BP1. Nat. Med. 7, 1128–1132 (2001).
Leonardsson, G. et al. Nuclear receptor co-repressor RIP140 regulates fat accumulation. Proc. Natl Acad. Sci. USA 101, 8437–8442 (2004).
Picard, F. et al. SRC-1 and TIF2 control energy balance between white and brown adipose tissues. Cell 111, 931–941 (2002).
Bonet, M. L., Oliver, P. & Palou, A. Pharmacological and nutritional agents promoting browning of white adipose tissue. Biochim. Biophys. Acta 1831, 969–985 (2013).
Cao, W. et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol. Cell Biol. 24, 3057–3067 (2004).
Martinez-deMena, R. & Obregón, M. J. Insulin increases the adrenergic stimulation of 5′ deiodinase activity and mRNA expression in rat brown adipocytes; role of MAPK and PI3K. J. Mol. Endocrinol. 34, 139–151 (2005).
Muller, T. D. et al. p62 links β-adrenergic input to mitochondrial function and thermogenesis. J. Clin. Invest 123, 469–478 (2013).
Ye, L. et al. Fat cells directly sense temperature to activate thermogenesis. Proc. Natl Acad. Sci. USA 110, 12480–12485 (2013).
Nedergaard, J. & Cannon, B. UCP1 mRNA does not produce heat. Biochim. Biophys. Acta 1831, 943–949 (2013).
Puigserver, P. et al. A cold-inducible co-activator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 (1998).
Hondares, E. et al. Thiazolidinediones and rexinoids induce peroxisome proliferator-activated receptor-co-activator (PGC)-1α gene transcription: an autoregulatory loop controls PGC-1α expression in adipocytes via peroxisome proliferator-activated receptor-γ co-activation. Endocrinology 147, 2829–2838 (2006).
Hondares, E. et al. Peroxisome proliferator-activated receptor α (PPARα) induces PPARγ co-activator 1α (PGC-1α) gene expression and contributes to thermogenic activation of brown fat: involvement of PRDM16. J. Biol. Chem. 286, 43112–43122 (2011).
Wang, Y. X. et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell 113, 159–170 (2003).
Alvarez, R. et al. A novel regulatory pathway of brown fat thermogenesis. Retinoic acid is a transcriptional activator of the mitochondrial uncoupling protein gene. J. Biol. Chem. 270, 5666–5673 (1995).
Mercader, J. et al. Remodeling of white adipose tissue after retinoic acid administration in mice. Endocrinology 147, 5325–5332 (2006).
Kiefer, F. W. et al. Retinaldehyde dehydrogenase 1 regulates a thermogenic programme in white adipose tissue. Nat. Med. 18, 918–925 (2012).
Pan, D., Fujimoto, M., Lopes, A. & Wang, Y. X. Twist-1 is a PPARδ-inducible, negative-feedback regulator of PGC-1α in brown fat metabolism. Cell 137, 73–86 (2009).
Seale, P. et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 6, 38–54 (2007).
Kajimura, S., Seale, P. & Spiegelman, B. M. Transcriptional control of brown fat development. Cell Metab. 11, 257–262 (2010).
Seale, P. et al. Prdm16 determines the thermogenic programme of subcutaneous white adipose tissue in mice. J. Clin. Invest. 121, 96–105 (2011).
Wilson-Fritch, L. et al. Mitochondrial remodeling in adipose tissue associated with obesity and treatment with rosiglitazone. J. Clin. Invest. 114, 1281–1289 (2004).
Ohno, H., Shinoda, K., Spiegelman, B. M. & Kajimura, S. PPARγ agonists induce a white-to-brown fat conversion through stabilization of PRDM16 protein. Cell Metab. 15, 395–404 (2012).
Trajkovski, M., Ahmed, K., Esau, C. C. & Stoffel, M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 14, 1330–1335 (2012).
Liu, W. et al. miR-133a regulates adipocyte browning in vivo. PLoS Genet. 9, e1003626 (2013).
Sun, L. et al. Mir193b-365 is essential for brown fat differentiation. Nat. Cell Biol. 13, 958–965 (2011).
Chen, Y. et al. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 4, 1769 (2013).
Mori, M., Nakagami, H., Rodriguez-Araujo, G., Nimura, K. & Kaneda, Y. Essential role for miR-196a in brown adipogenesis of white fat progenitor cells. PLoS Biol. 10, e1001314 (2012).
Galmozzi, A. et al. Inhibition of class I histone deacetylases unveils a mitochondrial signature and enhances oxidative metabolism in skeletal muscle and adipose tissue. Diabetes 62, 732–742 (2013).
Villanueva, C. J. et al. Adipose subtype-selective recruitment of TLE3 or Prdm16 by PPARγ specifies lipid storage versus thermogenic gene programmes. Cell Metab. 17, 423–435 (2013).
Qiang, L. et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 150, 620–632 (2012).
Rodgers, J. T. et al. Nutrient control of glucose homeostasis through a complex of PGC-1α and SIRT1. Nature 434, 113–118 (2005).
Canto, C. et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature 458, 1056–1060 (2009).
Jager, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Hawley, S. A. et al. Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11, 554–565 (2010).
Park, S. J. et al. Resveratrol ameliorates aging-related metabolic phenotypes by inhibiting cAMP phosphodiesterases. Cell 148, 421–433 (2012).
Lagouge, M. et al. Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1α. Cell 127, 1109–1122 (2006).
Gaidhu, M. P. et al. Chronic AMP-kinase activation with AICAR reduces adiposity by remodeling adipocyte metabolism and increasing leptin sensitivity. J. Lipid Res. 52, 1702–1711 (2011).
Vila-Bedmar, R., Lorenzo, M. & Fernandez-Veledo, S. Adenosine 5′-monophosphate-activated protein kinase-mammalian target of rapamycin cross talk regulates brown adipocyte differentiation. Endocrinology 151, 980–992 (2010).
Hawley, S. A. et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science 336, 918–922 (2012).
Haemmerle, G. et al. ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–1085 (2011).
Haemmerle, G. et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science 312, 734–737 (2006).
Ahmadian, M. et al. Desnutrin/ATGL is regulated by AMPK and is required for a brown adipose phenotype. Cell Metab. 13, 739–748 (2011).
Mottillo, E. P., Bloch, A. E., Leff, T. & Granneman, J. G. Lipolytic products activate peroxisome proliferator-activated receptor (PPAR) α and δ in brown adipocytes to match fatty acid oxidation with supply. J. Biol. Chem. 287, 25038–25048 (2012).
Nishino, N. et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J. Clin. Invest. 118, 2808–2821 (2008).
Toh, S. Y. et al. Upregulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS ONE 3, e2890 (2008).
Sawada, T. et al. Perilipin overexpression in white adipose tissue induces a brown fat-like phenotype. PLoS ONE 5, e14006 (2010).
Hotamisligil, G. S. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell 140, 900–917 (2010).
Singh, R. et al. Autophagy regulates lipid metabolism. Nature 458, 1131–1135 (2009).
Singh, R. et al. Autophagy regulates adipose mass and differentiation in mice. J. Clin. Invest 119, 3329–3339 (2009).
Settembre, C. et al. TFEB controls cellular lipid metabolism through a starvation-induced autoregulatory loop. Nat. Cell Biol. 15, 647–658 (2013).
Heeren, J. & Munzberg, H. Novel aspects of brown adipose tissue biology. Endocrinol. Metab. Clin. North Am. 42, 89–107 (2013).
Nguyen, K. D. et al. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature 480, 104–108 (2011).
Lumeng, C. N., Bodzin, J. L. & Saltiel, A. R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Invest. 117, 175–184 (2007).
Madsen, L. et al. UCP1 induction during recruitment of brown adipocytes in white adipose tissue is dependent on cyclooxygenase activity. PLoS ONE 5, e11391 (2010).
Vegiopoulos, A. et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science 328, 1158–1161 (2010).
Qian, S. W. et al. BMP4-mediated brown fat-like changes in white adipose tissue alter glucose and energy homeostasis. Proc. Natl Acad. Sci. USA 110, E798–E807 (2013).
Fisher, F. M. et al. FGF21 regulates PGC-1α and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 26, 271–281 (2012).
Lodhi, I. J. et al. Inhibiting adipose tissue lipogenesis reprogrammes thermogenesis and PPARγ activation to decrease diet-induced obesity. Cell Metab. 16, 189–201 (2012).
Bartelt, A. et al. Effects of adipocyte lipoprotein lipase on de novo lipogenesis and white adipose tissue browning. Biochim. Biophys. Acta 1831, 934–942 (2013).
Dutchak, P. A. et al. Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148, 556–567 (2012).
Hondares, E. et al. Hepatic FGF21 expression is induced at birth via PPARα in response to milk intake and contributes to thermogenic activation of neonatal brown fat. Cell Metab. 11, 206–212 (2010).
Kim, K. H. et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 19, 83–92 (2013).
Tseng, Y. H. et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 454, 1000–1004 (2008).
Whittle, A. J. et al. BMP8B increases brown adipose tissue thermogenesis through both central and peripheral actions. Cell 149, 871–885 (2012).
Tang, Q. Q., Otto, T. C. & Lane, M. D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl Acad. Sci. USA 101, 9607–9611 (2004).
Bowers, R. R. & Lane, M. D. A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 6, 385–389 (2007).
Morrison, S. F., Madden, C. J. & Tupone, D. Central control of brown adipose tissue thermogenesis. Front. Endocrinol. 3, 00005 (2012).
Di Marzo, V. & Matias, I. Endocannabinoid control of food intake and energy balance. Nat. Neurosci. 8, 585–589 (2005).
Zeltser, L. M., Seeley, R. J. & Tschöp, M. H. Synaptic plasticity in neuronal circuits regulating energy balance. Nat. Neurosci. 15, 1336–1342 (2012).
Yi, C. X. & Tschöp, M. H. Brain-gut-adipose-tissue communication pathways at a glance. Dis. Model. Mech. 5, 583–587 (2012).
Cao, L. et al. White to brown fat phenotypic switch induced by genetic and environmental activation of a hypothalamic–adipocyte axis. Cell Metab. 14, 324–338 (2011).
Paedersen, B. K. & Febbraio, M. A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 8, 457–465 (2012).
Bordicchia, M. et al. Cardiac natriuretic peptides act via p38 MAPK to induce the brown fat thermogenic programme in mouse and human adipocytes. J. Clin. Invest. 122, 1022–1036 (2012).
Mitschke, M. M. et al. Increased cGMP promotes healthy expansion and browning of white adipose tissue. FASEB J. 27, 1621–1630 (2013).
Sun, Z. Cardiovascular responses to cold exposure. Front. Biosci. (Elite Ed) 2, 495–503 (2010).
Handschin, C. & Spiegelman, B. M. The role of exercise and PGC1α in inflammation and chronic disease. Nature 454, 463–469 (2008).
Bostrom, P. et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 481, 463–468 (2012).
Zhang, C. et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 55, 183–193 (2012).
Shan, T., Liang, X., Bi, P. & Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α–Fndc5 pathway in muscle. FASEB J. 27, 1981–1989 (2013).
Ruas, J. L. et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151, 1319–1331 (2012).
Huh, J. Y. et al. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism 61, 1725–1738 (2012).
Lecker, S. H. et al. Expression of the irisin precursor FNDC5 in skeletal muscle correlates with aerobic exercise performance in patients with heart failure. Circ. Heart Fail. 5, 812–818 (2012).
Moreno-Navarrete, J. M. et al. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 98, E769–E778 (2013).
Raschke S. et al. Evidence against a Beneficial Effect of Irisin in Humans. PLoS ONE 8, e73680 (2013).
Staiger, H. et al. Common genetic variation in the human FNDC5 locus, encoding the novel muscle-derived 'browning' factor irisin, determines insulin sensitivity. PLoS ONE 8, e61903 (2013).
Bartelt, A., Merkel, M. & Heeren, J. A new, powerful player in lipoprotein metabolism: brown adipose tissue. J. Mol. Med. (Berl.) 90, 887–893 (2012).
Bartelt, A. et al. Brown adipose tissue activity controls triglyceride clearance. Nat. Med. 17, 200–205 (2011).
Stanford, K. I. et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Invest. 123, 215–223 (2013).
Liu, X. et al. Brown adipose tissue transplantation improves whole-body energy metabolism. Cell Res. 23, 851–854 (2013).
Kim, J. H., Bachmann, R. A. & Chen, J. Interleukin-6 and insulin resistance. Vitam. Horm. 80, 613–633 (2009).
Guerra, C., Koza, R. A., Yamashita, H., Walsh, K. & Kozak, L. P. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J. Clin. Invest. 102, 412–420 (1998).
Bachmanov, A. A., Reed, D. R., Tordoff, M. G., Price, R. A. & Beauchamp, G. K. Nutrient preference and diet-induced adiposity in C57BL/6ByJ and 129P3/J mice. Physiol. Behav. 72, 603–613 (2001).
Tran, T. T., Yamamoto, Y., Gesta, S. & Kahn, C. R. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 7, 410–420 (2008).
Schulz, T. J. et al. Brown-fat paucity due to impaired BMP signalling induces compensatory browning of white fat. Nature 495, 379–383 (2013).
Bal, N. C. et al. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat. Med. 18, 1575–1579 (2012).
Chechi, K., Blanchard, P. G., Mathieu, P., Deshaies, Y. & Richard, D. Brown fat like gene expression in the epicardial fat depot correlates with circulating HDL-cholesterol and triglycerides in patients with coronary artery disease. Int. J. Cardiol. 167, 2264–2270 (2013).
Orava, J. et al. Different metabolic responses of human brown adipose tissue to activation by cold and insulin. Cell Metab. 14, 272–279 (2011).
Ouellet, V. et al. Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans. J. Clin. Invest. 122, 545–552 (2012).
Orava, J. et al. Blunted metabolic responses to cold and insulin stimulation in brown adipose tissue of obese humans. Obesity (Silver Spring) http://dx.doi.org/10.1002/oby.20456.
Nisoli, E. et al. Tumour necrosis factor α mediates apoptosis of brown adipocytes and defective brown adipocyte function in obesity. Proc. Natl Acad. Sci. USA 97, 8033–8038 (2000).
Miranda, S., González-Rodriguez, A., Revuelta-Cervantes, J., Rondinone, C. M. & Valverde, A. M. Beneficial effects of PTP1B deficiency on brown adipocyte differentiation and protection against apoptosis induced by pro- and anti-inflammatory stimuli. Cell Signal. 22, 645–659 (2010).
Bagchi, M. et al. Vascular endothelial growth factor is important for brown adipose tissue development and maintenance. FASEB J. 27, 3257–3271 (2013).
Xu, X. et al. Exercise ameliorates high-fat diet-induced metabolic and vascular dysfunction, and increases adipocyte progenitor cell population in brown adipose tissue. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R1115–R1125 (2011).
Russell, A. P. et al. Brown adipocyte progenitor population is modified in obese and diabetic skeletal muscle. Int. J. Obes. (Lond.) 36, 155–158 (2012).
Hu, H. H., Smith, D. L. Jr., Nayak, K. S., Goran, M. I. & Nagy, T. R. Identification of brown adipose tissue in mice with fat-water IDEAL-MRI. J. Magn. Reson. Imaging 31, 1195–1202 (2010).
Chen, Y. I. et al. Anatomical and functional assessment of brown adipose tissue by magnetic resonance imaging. Obesity (Silver Spring) 20, 1519–1526 (2012).
Iris Chen, Y. C. et al. Measurement of human brown adipose tissue volume and activity using anatomic MR imaging and functional MR imaging. J. Nucl. Med. 54, 1584–1587 (2013).
Vliegenthart, R. et al. Dual-energy CT of the heart. AJR Am. J. Roentgenol. 199 (Suppl. 5), S54–S63 (2012).
Bruns, O. T. et al. Real-time magnetic resonance imaging and quantification of lipoprotein metabolism in vivo using nanocrystals. Nat. Nanotechnol. 4, 193–201 (2009).
Heeren, J. & Bruns, O. Nanocrystals, a new tool to study lipoprotein metabolism and atherosclerosis. Curr. Pharm. Biotechnol. 13, 365–372 (2012).
Acknowledgements
The authors thank all members of the Heeren lab for continuous support and enjoyable atmosphere, and are grateful to Rudolph Reimer for providing pictures taken by electron microscopy. A. Bartelt is supported by a Deutsche Forschungsgemeinschaft Research Fellowship (BA 4925/1-1). J. Heeren is supported by a grant from the Fondation Leducq—Triglyceride Metabolism in Obesity and Cardiovascular Disease and by EU FP7 project RESOLVE (FP7-HEALTH-2012-305707).
Author information
Authors and Affiliations
Contributions
Both authors contributed equally to all aspects of this article.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bartelt, A., Heeren, J. Adipose tissue browning and metabolic health. Nat Rev Endocrinol 10, 24–36 (2014). https://doi.org/10.1038/nrendo.2013.204
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrendo.2013.204
This article is cited by
-
Intestinal dual-specificity phosphatase 6 regulates the cold-induced gut microbiota remodeling to promote white adipose browning
npj Biofilms and Microbiomes (2024)
-
Telmisartan and candesartan promote browning of white adipose tissue and reverse fatty liver changes in high fat diet fed male albino rats
Naunyn-Schmiedeberg's Archives of Pharmacology (2024)
-
Metformin treatment of juvenile mice alters aging-related developmental and metabolic phenotypes in sex-dependent and sex-independent manners
GeroScience (2024)
-
Pulling the trigger: Noncoding RNAs in white adipose tissue browning
Reviews in Endocrine and Metabolic Disorders (2024)
-
Obesity causes mitochondrial fragmentation and dysfunction in white adipocytes due to RalA activation
Nature Metabolism (2024)