Key Points
-
Nicotine dependence is a major public health problem. Food and Drug Administration (FDA)-approved medications include nicotine-replacement therapies, bupropion and varenicline. Yet, only about 1 in 4 smokers are able to quit and remain abstinent for 1 year or more.
-
Significant progress in elucidating the molecular neurobiology of nicotine dependence has identified several mechanistic targets for translational research in medication development. These include, but are not limited to, dopaminergic, GABA (g-aminobutyric acid)-mediated, glutamatergic and opioid processes; intracellular signalling molecules (CREB (cyclic AMP response element binding), ΔFosB); and nicotinic cholinergic receptors.
-
The lack of validated animal and human laboratory paradigms that have demonstrated predictive clinical validity is a major obstacle to the development of improved medications for nicotine dependence. Scientists in academic institutions and industry can and should contribute to medication development for drug dependence by addressing this critical gap.
-
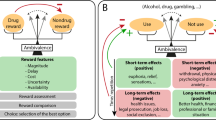
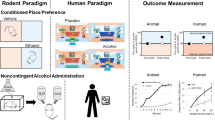
In animals and humans, the reinforcing value of drugs of abuse, including nicotine, can be assessed using self-administration models. Reward, a concept related to but different from reinforcement, can be measured in animals using conditioned place preference and intracranial self-stimulation paradigms. In humans, drug reward is measured using self-report assessments.
-
The negative-reinforcing effects of drugs of abuse focus on the alleviation of physiological, affective and neurocognitive signs of drug withdrawal in dependent subjects. Although many models exist to assess these variables, few have been tested for predictive clinical validity for medication screening.
-
Relapse is a central concept in drug dependence. In animals this is typically assessed using reinstatement models. Novel paradigms are also being developed for assessing medication effects on relapse in the human laboratory setting.
-
Animal and human laboratory models require further validation if they are to be used to optimize medication development. Ideally, these models should provide an early indication of the likely efficacy of a medication before proceeding to more costly clinical trials. One opportunity to address model validation would be to examine medications with proven efficacy for smoking cessation across each of the animal and human laboratory models.
Abstract
A major obstacle to the development of medications for nicotine dependence is the lack of animal and human laboratory models with sufficient predictive clinical validity to support the translation of knowledge from laboratory studies to clinical research. This Review describes the animal and human laboratory paradigms commonly used to investigate the pathophysiology of nicotine dependence, and proposes how their predictive validity might be determined and improved, thereby enhancing the development of new medications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
van den Brink, W. & Haasen, C. Evidenced-based treatment of opioid-dependent patients. Can. J. Psychiatry 51, 635–646 (2006).
Kreek, M. J., LaForge, K. S. & Butelman, E. Pharmacotherapy of addictions. Nature Rev. Drug Discovery 1, 710–726 (2002). An overview of pharmacotherapy and medication development for addictions, focusing on alcohol and illicit drug dependence.
Pouletty, P. Drug addictions: towards socially accepted and medically treatable diseases. Nature Rev. Drug Discovery 1, 731–736 (2002).
Schnoll, R. & Lerman, C. Current and emerging pharmacotherapies for treating tobacco dependence. Expert Opin. Emerg. Drugs 11, 429–444 (2006).
Perkins, K. A., Stitzer, M. & Lerman, C. Medication screen-ing for smoking cessation: a proposal for new method-ologies. Psychopharmacology 184, 628–636 (2006). A commentary that highlights the limitations in human screening of cessation medications and suggests novel methodologies.
Buisson, B. & Bertrand, D. Nicotine addiction: the possible role of functional upregulation. Trends Pharmacol. Sci. 23, 130–136 (2002).
Baker, T. B., Piper, M. E., McCarthy, D. E., Majeskie, M. R. & Fiore, M. C. Addiction motivation reformulated: an affective processing model of negative reinforcement. Psychol. Rev. 111, 33–51 (2004).
Bossert, J. M., Ghitza, U. E., Lu, L., Epstein, D. H. & Shaham, Y. Neurobiology of relapse to heroin and cocaine seeking: an update and clinical implications. Eur. J. Pharmacol. 526, 36–50 (2005).
Koob, G. F. The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction 101 (Suppl. 1), 23–30 (2006). A contemporary overview of CNS systems and behavioural processes that have been proposed to play a role in drug dependence.
Conklin, C. A. et al. The return to smoking: 1-year relapse trajectories among female smokers. Nicotine Tob. Res. 7, 533–540 (2005).
Perkins, K. A. Chronic tolerance to nicotine in humans and its relationship to tobacco dependence. Nicotine Tob. Res. 4, 405–422 (2002).
Perkins, K. A. et al. Quitting cigarette smoking produces minimal loss of chronic tolerance to nicotine. Psychopharmacology 158, 7–17 (2001).
Robinson, T. E. & Berridge, K. C. Incentive-sensitization and addiction. Addiction 96, 103–114 (2001).
Wise, R. A. Brain reward circuitry: insights from unsensed incentives. Neuron 36, 229–240 (2002).
Wise, R. A. Dopamine, learning and motivation. Nature Rev. Neurosci. 5, 483–494 (2004).
Hyman, S. E., Malenka, R. C. & Nestler, E. J. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu. Rev.Neurosci. 29, 565–598 (2006).
Hyman, S. E. Addiction: a disease of learning and memory. Am. J. Psychiatry 162, 1414–1422 (2005).
Kauer, J. A. Learning mechanisms in addiction: synaptic plasticity in the ventral tegmental area as a result of exposure to drugs of abuse. Annu. Rev. Physiol. 66, 447–475 (2004).
Redish, A. D. Addiction as a computational process gone awry. Science 306, 1944–1947 (2004).
Tobler, P. N., Fiorillo, C. D. & Schultz, W. Adaptive coding of reward value by dopamine neurons. Science 307, 1642–1645 (2005).
Vezina, P. Sensitization of midbrain dopamine neuron reactivity and the self-administration of psychomotor stimulant drugs. Neurosci. Biobehav. Rev. 27, 827–839 (2004).
Corrigall, W. A., Coen, K. M. & Adamson, K. L. Self-administered nicotine activates the mesolimbic dopamine system through the ventral tegmental area. Brain Res. 653, 278–284 (1994). This study was the first to use a model of nicotine self-administration by laboratory rats to investigate the CNS mechanisms of nicotine dependence.
Kalivas, P. W. & Volkow, N. D. The neural basis of addiction: a pathology of motivation and choice. Am. J. Psychiatry 162, 1403–1413 (2005).
Corrigall, W. A., Franklin, K. B., Coen, K. M. & Clarke, P. B. The mesolimbic dopaminergic system is implicated in the reinforcing effects of nicotine. Psychopharmacology 107, 285–289 (1992).
Picciotto, M. R. & Corrigall, W. A. Neuronal systems underlying behaviors related to nicotine addiction: neural circuits and molecular genetics. J. Neurosci. 22, 3338–3341 (2002).
Nestler, E. J. Is there a common molecular pathway for addiction? Nature Neurosci. 8, 1445–1449 (2005).
Laviolette, S. R. & van der Kooy, D. The neurobiology of nicotine addiction: bridging the gap from molecules to behaviour. Nature Rev. Neurosci. 5, 55–65 (2004). This paper illustrates how complex interactions within a single brain region, such as the ventral tegmental area, may mediate both positive and aversive effects of nicotine.
Jose Lanca, A., Sanelli, T. R. & Corrigall, W. A. Nicotine-induced fos expression in the pedunculopontine mesencephalic tegmentum in the rat. Neuropharmacology 39, 2808–2817 (2000).
Klink, R., de Kerchove d'Exaerde, A., Zoli, M. & Changeux, J. P. Molecular and physiological diversity of nicotinic acetylcholine receptors in the midbrain dopaminergic nuclei. J. Neurosci. 21, 1452–1463 (2001).
Chao, J. & Nestler, E. J. Molecular neurobiology of drug addiction. Annu. Rev. Med. 55, 113–132 (2004).
Holmes, A., Heilig, M., Rupniak, N. M., Steckler, T. & Griebel, G. Neuropeptide systems as novel therapeutic targets for depression and anxiety disorders. Trends Pharmacol. Sci. 24, 580–588 (2003).
Volkow, N. D. & Li, T. K. Drug addiction: the neurobiology of behaviour gone awry. Nature Rev. Neurosci. 5, 963–970 (2004).
Harris, G. C., Wimmer, M. & Aston-Jones, G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature 437, 556–559 (2005).
Harris, G. C. & Aston-Jones, G. Arousal and reward: a dichotomy in orexin function. Trends Neurosci. 29, 571–577 (2006).
Funk, C. K., O'Dell, L. E., Crawford, E. F. & Koob, G. F. Corticotropin-releasing factor within the central nucleus of the amygdala mediates enhanced ethanol self-administration in withdrawn, ethanol-dependent rats. J. Neurosci. 26, 11324–11332 (2006).
Dani, J. A. & Bertrand, D. Nicotinic acetylcholine receptors and nicotinic cholinergic mechanisms of the central nervous system. Annu. Rev. Pharmacol. Toxicol. 47, 699–729 (2007).
Gotti, C., Zoli, M. & Clementi, F. Brain nicotinic acetylcholine receptors: native subtypes and their relevance. Trends Pharmacol. Sci. 27, 482–491 (2006). A thorough recent summary of knowledge regarding the composition, distribution and function of nicotinic cholinergic receptors.
Roth, B. L., Sheffler, D. J. & Kroeze, W. K. Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nature Rev. Drug Discov. 3, 353–359 (2004).
Sanchis-Segura, C. & Spanagel, R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addict. Biol. 11, 2–38 (2006).
Everitt, B. J. & Robbins, T. W. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 8, 1481–1489 (2005).
Goldberg, S. R., Spealman, R. D. & Goldberg, D. M. Persistent behavior at high rates maintained by intravenous self-administration of nicotine. Science 214, 573–575 (1981).
Caggiula, A. R. et al. Cue dependency of nicotine self-administration and smoking. Pharmacol. Biochem. Behav. 70, 515–530 (2001).
Corrigall, W. A., Coen, K. M., Zhang, J. & Adamson, L. Pharmacological manipulations of the pedunculopontine tegmental nucleus in the rat reduce self-administration of both nicotine and cocaine. Psychopharmacology 160, 198–205 (2002).
Lanca, A. J., Adamson, K. L., Coen, K. M., Chow, B. L. & Corrigall, W. A. The pedunculopontine tegmental nucleus and the role of cholinergic neurons in nicotine self-administration in the rat: a correlative neuroanatomical and behavioral study. Neuroscience 96, 735–742 (2000).
Belluzzi, J. D., Wang, R. & Leslie, F. M. Acetaldehyde enhances acquisition of nicotine self-administration in adolescent rats. Neuropsychopharmacology 30, 705–712 (2005).
Villegier, A. S. et al. Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology 31, 1704–1713 (2006).
Sharpe, A. L. & Samson, H. H. Effect of naloxone on appetitive and consummatory phases of ethanol self-administration. Alcohol. Clin. Exp. Res. 25, 1006–1011 (2001).
Chen, S. A. et al. Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology 31, 2692–2707 (2006).
Stromberg, M. F., Meister, S., Volpicelli, J. R. & Ulm, R. R. Morphine enhances selection of both sucrose and ethanol in a two-bottle test. Alcohol 14, 55–62 (1997).
Ferraro, T. N. et al. Confirmation of a major QTL influencing oral morphine intake in C57 and DBA mice using reciprocal congenic strains. Neuropsychopharmacology 30, 742–746 (2005).
Ray, R. et al. Association of OPRM1 A118G variant with the relative reinforcing value of nicotine. Psychopharmacology 188, 355–363 (2006).
Perkins, K. A., Fonte, C., Meeker, J., White, W. & Wilson, A. The discriminative stimulus and reinforcing effects of nicotine in humans following nicotine pretreatment. Behav. Pharmacol. 12, 35–44 (2001).
Perkins, K. A. et al. Acute nicotine reinforcement, but not chronic tolerance, predicts withdrawal and relapse after quitting smoking. Health Psychol. 21, 332–339 (2002).
Rukstalis, M. et al. Naltrexone reduces the relative reinforcing value of nicotine in a cigarette smoking choice paradigm. Psychopharmacology 180, 41–48 (2005).
Bickel, W. K., DeGrandpre, R. J. & Higgins, S. T. The behavioral economics of concurrent drug reinforcers: a review and reanalysis of drug self-administration research. Psychopharmacology 118, 250–259 (1995).
Kenny, P. J. & Markou, A. Nicotine self-administration acutely activates brain reward systems and induces a long-lasting increase in reward sensitivity. Neuropsychopharmacology 31, 1203–1211 (2006).
Kornetsky, C., Esposito, R. U., McLean, S. & Jacobson, J. O. Intracranial self-stimulation thresholds: a model for the hedonic effects of drugs of abuse. Arch. Gen. Psychiatry 36, 289–292 (1979).
Kenny, P. J., Chen, S. A., Kitamura, O., Markou, A. & Koob, G. F. Conditioned withdrawal drives heroin consumption and decreases reward sensitivity. J. Neurosci. 26, 5894–5900 (2006).
Shiffman, S., Ferguson, S. G. & Gwaltney, C. J. Immediate hedonic response to smoking lapses: relationship to smoking relapse, and effects of nicotine replacement therapy. Psychopharmacology 184, 608–618 (2006).
Rose, J. E. Nicotine and nonnicotine factors in cigarette addiction. Psychopharmacology 184, 274–285 (2006). This review considers the crucial contributions of conditioned reinforcement in nicotine dependence and highlights key non-nicotine factors that contribute to dependence.
Conklin, C. A. Environments as cues to smoke: implications for human extinction-based research and treatment. Exp. Clin. Psychopharm. 14, 12–19 (2006).
Carter, B. L. & Tiffany, S. T. Meta-analysis of cue-reactivity in addiction research. Addiction 94, 327–340 (1999).
Goldstein, R. Z. et al. Role of the anterior cingulate and medial orbitofrontal cortex in processing drug cues in cocaine addiction. Neuroscience 144, 1153–1159 (2007).
Brody, A. L. et al. Brain metabolic changes during cigarette craving. Arch. Gen. Psychiatry 59, 1162–1172 (2002).
McBride, D., Barrett, S. P., Kelly, J. T., Aw, A. & Dagher, A. Effects of expectancy and abstinence on the neural response to smoking cues in cigarette smokers: an fMRI study. Neuropsychopharmacology 31, 2728–2738 (2006).
Due, D. L., Huettel, S. A., Hall, W. G. & Rubin, D. C. Activation in mesolimbic and visuospatial neural circuits elicited by smoking cues: evidence from functional magnetic resonance imaging. Am. J. Psychiatry 159, 954–960 (2002).
Marissen, M. A. et al. Attentional bias predicts heroin relapse following treatment. Addiction 101, 1306–1312 (2006).
Waters, A. J. et al. Attentional bias predicts outcome in smoking cessation. Health Psychol. 22, 378–387 (2003).
Le Foll, B. & Goldberg, S. R. Nicotine induces conditioned place preferences over a large range of doses in rats. Psychopharmacology 178, 481–492 (2005).
Bardo, M. T. & Bevins, R. A. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology 153, 31–43 (2000).
Walters, C. L. & Blendy, J. A. Different requirements for cAMP response element binding protein in positive and negative reinforcing properties of drugs of abuse. J. Neurosci. 21, 9438–9444 (2001).
Walters, C. L., Cleck, J. N., Kuo, Y. C. & Blendy, J. A. Mu-opioid receptor and CREB activation are required for nicotine reward. Neuron 46, 933–943 (2005).
Donny, E. C. et al. Nicotine self-administration in rats on a progressive ratio schedule of reinforcement. Psychopharmacology 147, 135–142 (1999).
LeSage, M. G., Burroughs, D., Dufek, M., Keyler, D. E. & Pentel, P. R. Reinstatement of nicotine self-administration in rats by presentation of nicotine-paired stimuli, but not nicotine priming. Pharmacol. Biochem. Behav. 79, 507–513 (2004).
Liu, X. et al. Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology 184, 417–425 (2006).
Phillips, A. G. & Fibiger, H. C. Role of reward and enhancement of conditioned reward in persistence of responding for cocaine. Behav. Pharmacol. 1, 269–282 (1990).
Donny, E. C. et al. Operant responding for a visual reinforcer in rats is enhanced by noncontingent nicotine: implications for nicotine self-administration and reinforcement. Psychopharmacology 169, 68–76 (2003).
Palmatier, M. I., Liu, X., Caggiula, A. R., Donny, E. C. & Sved, A. F. The role of nicotinic acetylcholine receptors in the primary reinforcing and reinforcement-enhancing effects of nicotine. Neuropsychopharmacology 32, 1098–1108 (2007).
Malin, D. H. et al. Analog of neuropeptide FF attenuates morphine abstinence syndrome. Peptides 12, 1011–1014 (1991).
Pinel, J. P. Alcohol withdrawal seizures: implications of kindling. Pharmacol. Biochem. Behav. 13 (Suppl. 1), 225–231 (1980).
Malin, D. H. et al. The nicotinic antagonist mecamylamine precipitates nicotine abstinence syndrome in the rat. Psychopharmacology 115, 180–184 (1994).
Grabus, S. D., Martin, B. R. & Imad Damaj, M. Nicotine physical dependence in the mouse: involvement of the α7 nicotinic receptor subtype. Eur. J. Pharmacol. 515, 90–93 (2005).
Maxwell, C. R. et al. Ketamine produces lasting disruptions in encoding of sensory stimuli. J. Pharmacol. Exp. Ther. 316, 315–324 (2006).
Siegel, S. J. et al. Effects of strain, novelty, and NMDA blockade on auditory-evoked potentials in mice. Neuropsychopharmacology 28, 675–682 (2003).
Siegel, S. J. et al. Monoamine reuptake inhibition and nicotine receptor antagonism reduce amplitude and gating of auditory evoked potentials. Neuroscience 133, 729–738 (2005).
Phillips, J., Ehrlichman, R. & Siegel, S. Mecamylamine blocks nicotine-induced enhancement of the P20 auditory event related potential and evoked gamma. Neuroscience 144, 1314–1323 (2007).
Shoaib, M. & Bizarro, L. Deficits in a sustained attention task following nicotine withdrawal in rats. Psychopharmacology 178, 211–222 (2005).
Levine, A. J. et al. The effect of recent stimulant use on sustained attention in HIV-infected adults. J. Clin. Exp. Neuropsychology 28, 29–42 (2006).
Levin, E. D. et al. Transdermal nicotine effects on attention. Psychopharmacology 140, 135–141 (1998).
Davis, J. A. & Gould, T. J. Atomoxetine reverses nicotine withdrawal-associated deficits in contextual fear conditioning. Neuropsychopharmacology 17 Jan 2007 (doi:10.1038/sj.npp.1301315).
Davis, J. A., James, J. R., Siegel, S. J. & Gould, T. J. Withdrawal from chronic nicotine administration impairs contextual fear conditioning in C57BL/6 mice. J. Neurosci. 25, 8708–8713 (2005).
Woods, S. C., Fay, J., Sage, J. R. & Anagnostaras, S. G. Cocaine and Pavlovian fear conditioning: dose–effect analysis. Behav. Brain Res. 176, 244–250 (2007).
Piasecki, T. M., Fiore, M. C. & Baker, T. B. Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J. Abnorm. Psychol. 107, 238–251 (1998).
Hurt, R. D. et al. A comparison of sustained-release bupropion and placebo for smoking cessation. N. Engl. J. Med. 337, 1195–1202 (1997).
Hughes, J., Stead, L. & Lancaster, T. Antidepressants for smoking cessation. Cochrane Database Syst Rev. 1, CD000031 (2007).
Lin, D., Koob, G. F. & Markou, A. Differential effects of withdrawal from chronic amphetamine or fluoxetine administration on brain stimulation reward in the rat — interactions between the two drugs. Psychopharmacology 145, 283–294 (1999).
Markou, A. & Koob, G. F. Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4, 17–26 (1991).
Kenny, P. J. & Markou, A. Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J. Neurosci. 25, 6208–6212 (2005).
Davis, M. Neurochemical modulation of sensory–motor reactivity: acoustic and tactile startle reflexes. Neurosci. Biobehav. Rev. 4, 241–263 (1980).
Bradley, M. M., Cuthbert, B. N. & Lang, P. J. Startle and emotion: lateral acoustic probes and the bilateral blink. Psychophysiology 28, 285–295 (1991).
Braff, D. L., Geyer, M. A. & Swerdlow, N. R. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies. Psychopharmacology 156, 234–258 (2001).
Sinha, R., Fuse, T., Aubin, L. R. & O'Malley, S. S. Psychological stress, drug-related cues and cocaine craving. Psychopharmacology 152, 140–148 (2000).
Junghanns, K. et al. Impaired serum cortisol stress response is a predictor of early relapse. Alcohol Alcohol. 38, 189–193 (2003).
Capriles, N., Rodaros, D., Sorge, R. E. & Stewart, J. A role for the prefrontal cortex in stress- and cocaine-induced reinstatement of cocaine seeking in rats. Psychopharmacology 168, 66–74 (2003).
Erb, S., Shaham, Y. & Stewart, J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology 128, 408–412 (1996).
Kreibich, A. S. & Blendy, J. A. cAMP response element-binding protein is required for stress but not cocaine-induced reinstatement. J. Neurosci. 24, 6686–6692 (2004).
Katz, J. L. & Higgins, S. T. The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168, 21–30 (2003).
Shaham, Y., Shalev, U., Lu, L., De Wit, H. & Stewart, J. The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology 168, 3–20 (2003).
Kippin, T. E., Fuchs, R. A. & See, R. E. Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology 187, 60–67 (2006).
Brandon, T. H., Tiffany, S. T., Obremski, K. M. & Baker, T. B. Postcessation cigarette use: the process of relapse. Addict. Behav. 15, 105–114 (1990).
Shiffman, S. et al. Progression from a smoking lapse to relapse: prediction from abstinence violation effects, nicotine dependence, and lapse characteristics. J. Consult. Clin. Psychol. 64, 993–1002 (1996).
Chornock, W. M., Stitzer, M. L., Gross, J. & Leischow, S. Experimental model of smoking re-exposure: effects on relapse. Psychopharmacology 108, 495–500 (1992).
Juliano, L. M., Donny, E. C., Houtsmuller, E. J. & Stitzer, M. L. Experimental evidence for a causal relationship between smoking lapse and relapse. J. Abnorm. Psychol. 115, 166–173 (2006).
McKee, S. A., Krishnan-Sarin, S., Shi, J., Mase, T. & O'Malley, S. S. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology 189, 201–210 (2006).
Smith, J. W. et al. Ligands selective for α4β2 but not α3β4 or α7 nicotinic receptors generalise to the nicotine discriminative stimulus in the rat. Psychopharmacology 190, 157–170 (2007).
Stolerman, I. P., Garcha, H. S., Pratt, J. A. & Kumar, R. Role of training dose in discrimination of nicotine and related compounds by rats. Psychopharmacology 84, 413–419 (1984).
Perkins, K. A., DiMarco, A., Grobe, J. E., Scierka, A. & Stiller, R. L. Nicotine discrimination in male and female smokers. Psychopharmacology 116, 407–413 (1994).
Perkins, K. A. Nicotine discrimination in men and women. Pharmacol. Biochem. Behav. 64, 295–299 (1999).
Rollema, H. et al. Pharmacological profile of the α(4)β(2) nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52, 985–994 (2007).
Kamien, J. B., Bickel, W. K., Hughes, J. R., Higgins, S. T. & Smith, B. J. Drug discrimination by humans compared to nonhumans: current status and future directions. Psychopharmacology 111, 259–270 (1993).
Egli, M. Can experimental paradigms and animal models be used to discover clinically effective medications for alcoholism? Addict. Biol. 10, 309–319 (2005).
O'Malley, S. S. & Froehlich, J. C. Advances in the use of naltrexone: an integration of preclinical and clinical findings. Recent Dev. Alcohol. 16, 217–245 (2003).
Fagerstrom, K. & Balfour, D. J. Neuropharmacology and potential efficacy of new treatments for tobacco dependence. Exp. Opin. Inv. Drug 15, 107–116 (2006).
Sofuoglu, M. & Kosten, T. R. Emerging pharmacological strategies in the fight against cocaine addiction. Exp. Opin. Emer. Drug 11, 91–98 (2006).
Sellers, E. M., Tyndale, R. F. & Fernandes, L. C. Decreasing smoking behaviour and risk through CYP2A6 inhibition. Drug Discov. Today 8, 487–493 (2003).
LeSage, M. G. et al. Effects of a nicotine conjugate vaccine on the acquisition and maintenance of nicotine self-administration in rats. Psychopharmacology 184, 409–416 (2006).
Maurer, P. & Bachmann, M. F. Therapeutic vaccines for nicotine dependence. Curr. Opin. Mol. Ther. 8, 11–16 (2006).
Silagy, C., Lancaster, T., Stead, L., Mant, D. & Fowler, G. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 3, CD000146 (2004).
Benowitz, N. L., Zevin, S. & Jacob, P. 3rd. Suppression of nicotine intake during ad libitum cigarette smoking by high-dose transdermal nicotine. J. Pharmacol. Exp. Ther. 287, 958–962 (1998).
LeSage, M. G., Keyler, D. E., Collins, G. & Pentel, P. R. Effects of continuous nicotine infusion on nicotine self-administration in rats: relationship between continuously infused and self-administered nicotine doses and serum concentrations. Psychopharmacology 170, 278–286 (2003).
O'Connor, K. A. & Roth, B. L. Finding new tricks for old drugs: an efficient route for public-sector drug discovery. Nature Rev. Drug Discov. 4, 1005–1014 (2005).
Uhl, G. R. Needed: mouse/human cross validation of reinstatement/relapse models (and drug reward models) to model human substance abuse vulnerability allelic variants. Psychopharmacology 168, 42–43 (2003).
Blendy, J. A. et al. Reduced nicotine reward in obesity: cross-comparison in human and mouse. Psychopharmacology 180, 306–315 (2005).
Fowler, J. S. et al. Inhibition of monoamine oxidase B in the brains of smokers. Nature 379, 733–736 (1996).
Guillem, K. et al. Monoamine oxidase A rather than monoamine oxidase B inhibition increases nicotine reinforcement in rats. Eur. J. Neurosci. 24, 3532–3540 (2006).
Guillem, K. et al. Monoamine oxidase inhibition dramatically increases the motivation to self-administer nicotine in rats. J. Neurosci. 25, 8593–8600 (2005).
Lasser, K. et al. Smoking and mental illness: a population-based prevalence study. JAMA 284, 2606–2610 (2000). A large-scale epidemiological study providing useful data on the prevalence of tobacco use in persons with psychiatric illness.
Corrigall, W. A. & Coen, K. M. Nicotine maintains robust self-administration in rats on a limited-access schedule. Psychopharmacology 99, 473–478 (1989). Now well-established as a research model, reliable nicotine self-administration was first reported in this publication. Together with reference 22, the paper shows the utility of models such as these in discovering CNS mechanisms in dependence.
Picciotto, M. R. et al. Acetylcholine receptors containing the β2 subunit are involved in the reinforcing properties of nicotine. Nature 391, 173–177 (1998).
Le Novere, N. & Changeux, J. P. Molecular evolution of the nicotinic acetylcholine receptor: an example of multigene family in excitable cells. J. Mol. Evol. 40, 155–172 (1995).
Horenstein, N. A., McCormack, T. J., Stokes, C., Ren, K. & Papke, R. L. Reversal of agonist selectivity by mutations of conserved amino acids in the binding site of nicotinic acetylcholine receptors. J. Biol. Chem. 282, 5899–5909 (2007).
Fonck, C. et al. Novel seizure phenotype and sleep disruptions in knock-in mice with hypersensitive α4* nicotinic receptors. J. Neurosci. 25, 11396–11411 (2005).
Cousins, M. S., Stamat, H. M. & de Wit, H. Acute doses of D-amphetamine and bupropion increase cigarette smoking. Psychopharmacology 157, 243–253 (2001).
Shoaib, M., Sidhpura, N. & Shafait, S. Investigating the actions of bupropion on dependence-related effects of nicotine in rats. Psychopharmacology 165, 405–412 (2003).
Teneggi, V. et al. Effect of sustained-release (SR) bupropion on craving and withdrawal in smokers deprived of cigarettes for 72 h. Psychopharmacology 183, 1–12 (2005).
O'Dell, L. E. et al. Extended access to nicotine self-administration leads to dependence: circadian measures, withdrawal measures, and extinction behavior in rats. J. Pharmacol. Exp. Ther. 320, 180–193 (2007).
Nader, M. A. & Woolverton, W. L. Effects of increasing the magnitude of an alternative reinforcer on drug choice in a discrete-trials choice procedure. Psychopharmacology 105, 169–174 (1991).
Borsook, D., Becerra, L. & Hargreaves, R. A role for fMRI in optimizing CNS drug development. Nature Rev. Drug Discov. 5, 411–424 (2006). A review of functional magnetic resonance imaging research and its application to the development of medications for CNS disorders.
Shoaib, M., Lowe, A. S. & Williams, S. C. Imaging localised dynamic changes in the nucleus accumbens following nicotine withdraw in rats. Neuroimage 22, 847–854 (2004).
Lowe, A. S., Williams, S. C., Symms, M. R., Stolerman, I. P. & Shoaib, M. Functional magnetic resonance neuroimaging of drug dependence: naloxone-precipitated morphine withdrawal. Neuroimage 17, 902–910 (2002).
Breiter, H. C. et al. Acute effects of cocaine on human brain activity and emotion. Neuron 19, 591–611 (1997).
Van Eldik, L. J., Koppal, T. & Watterson, D. M. Barriers to Alzheimer disease drug discovery and development in academia. Alzheimer Dis. Assoc. Disord. 16 (Suppl. 1), 18–28 (2002).
Eisenberg, R. S. & Nelson, R. R. Public vs. proprietary science: a fruitful tension? Acad. Med. 77, 1392–1399 (2002).
Prendergast, M. M. et al. Look beyond financial conflicts of interest in evaluating industry–academia collaborations in burden-of-illness and outcomes research studies in dermatology. J. Invest. Dermatol. 123, 452–454 (2004).
Chin-Dusting, J., Mizrahi, J., Jennings, G. & Fitzgerald, D. Outlook: finding improved medicines: the role of academic–industrial collaboration. Nature Rev. Drug Discov. 4, 891–897 (2005).
Kreek, M. J., Bart, G., Lilly, C., LaForge, K. S. & Nielsen, D. A. Pharmacogenetics and human molecular genetics of opiate and cocaine addictions and their treatments. Pharmacol. Rev. 57, 1–26 (2005).
Munafo, M. R., Shields, A. E., Berrettini, W. H., Patterson, F. & Lerman, C. Pharmacogenetics and nicotine addiction treatment. Pharmacogenomics 6, 211–223 (2005). An overview of emerging data on pharmacogenetic approaches to the treatment of nicotine dependence.
Liu, Q. R. et al. Pooled association genome scanning: validation and use to identify addiction vulnerability loci in two samples. Proc. Natl Acad. Sci. USA 102, 11864–11869 (2005).
Bierut, L. J. et al. Novel genes identified in a high-density genome wide association study for nicotine dependence. Hum. Mol. Genet. 16, 24–35 (2007). This paper illustrates the novel targets that are being uncovered by means of human genetics approaches.
Rutter, J. L. Symbiotic relationship of pharmacogenetics and drugs of abuse. AAPS J. 8, e174–e184 (2006). A review and commentary regarding the potential value of pharmacogenetics research to the treatment of drug abuse.
Cardon, L. R., Idury, R. M., Harris, T. J., Witte, J. S. & Elston, R. C. Testing drug response in the presence of genetic information: sampling issues for clinical trials. Pharmacogenetics 10, 503–510 (2000).
Kalivas, P. W. & McFarland, K. Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology 168, 44–56 (2003).
Wonnacott, S., Sidhpura, N. & Balfour, D. J. Nicotine: from molecular mechanisms to behaviour. Curr. Opin. Pharmacol. 5, 53–59 (2005). A summary of CNS circuits believed to be involved in nicotine dependence, derived largely from animal research.
Brody, A. L. Functional brain imaging of tobacco use and dependence. J. Psychiatr. Res. 40, 404–418 (2006). This paper summarizes brain regions that have been implicated in tobacco dependence with brain imaging studies in humans; it complements and expands on the circuits identified in reference 163.
Rose, J. E., Behm, F. M., Westman, E. C. & Kukovich, P. Precessation treatment with nicotine skin patch facilitates smoking cessation. Nicotine Tob. Res. 8, 89–101 (2006).
Brauer, L. H., Behm, F. M., Westman, E. C., Patel, P. & Rose, J. E. Naltrexone blockade of nicotine effects in cigarette smokers. Psychopharmacology 143, 339–346 (1999).
Perkins, K. A., Grobe, J. E., Stiller, R. L., Fonte, C. & Goettler, J. E. Nasal spray nicotine replacement suppresses cigarette smoking desire and behavior. Clin. Pharmacol. Ther. 52, 627–634 (1992).
Nemeth-Coslett, R., Henningfield, J. E., O'Keeffe, M. K. & Griffiths, R. R. Nicotine gum: dose-related effects on cigarette smoking and subjective ratings. Psychopharmacology 92, 424–430 (1987).
Perkins, K. A., Lerman, C., Keenan, J., Fonte, C. & Coddington, S. Rate of nicotine onset from nicotine replacement therapy and acute responses in smokers. Nicotine Tob. Res. 6, 501–507 (2004).
Shahan, T. A., Odum, A. L. & Bickel, W. K. Nicotine gum as a substitute for cigarettes: a behavioral economic analysis. Behav. Pharmacol. 11, 71–99 (2000).
Johnson, M. W. & Bickel, W. K. The behavioral economics of cigarette smoking: The concurrent presence of a substitute and an independent reinforcer. Behav. Pharmacol. 14, 137–144 (2003).
Tiffany, S. T., Cox, L. S. & Elash, C. A. Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J. Consult. Clin. Psychol. 68, 233–240 (2000).
Hurt, R. D. et al. Temporal effects of nicotine nasal spray and gum on nicotine withdrawal symptoms. Psychopharmacology 140, 98–104 (1998).
Rose, J. E., Behm, F. M. & Westman, E. C. Acute effects of nicotine and mecamylamine on tobacco withdrawal symptoms, cigarette reward and ad lib smoking. Pharmacol. Biochem. Behav. 68, 187–197 (2001).
Rose, J. E., Herskovic, J. E., Trilling, Y. & Jarvik, M. E. Transdermal nicotine reduces cigarette craving and nicotine preference. Clin. Pharmacol. Ther. 38, 450–456 (1985).
Brody, A. L. et al. Attenuation of cue-induced cigarette craving and anterior cingulate cortex activation in bupropion-treated smokers: a preliminary study. Psychiatry Res. 130, 269–281 (2004).
Shiffman, S. et al. The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology 148, 33–40 (2000).
Sutherland, G., Stapleton, J. A., Russell, M. A. & Feyerabend, C. Naltrexone, smoking behaviour and cigarette withdrawal. Psychopharmacology 120, 418–425 (1995).
Nemeth-Coslett, R. & Griffiths, R. R. Naloxone does not affect cigarette smoking. Psychopharmacology 89, 261–264 (1986).
Gorelick, D. A., Rose, J. & Jarvik, M. E. Effect of naloxone on cigarette smoking. J. Subst. Abuse 1, 153–159 (1988).
Epstein, A. M. & King, A. C. Naltrexone attenuates acute cigarette smoking behavior. Pharmacol. Biochem. Behav. 77, 29–37 (2004).
King, A. C. & Meyer, P. J. Naltrexone alteration of acute smoking response in nicotine-dependent subjects. Pharmacol. Biochem. Behav. 66, 563–572 (2000).
Wewers, M. E., Dhatt, R. & Tejwani, G. A. Naltrexone administration affects ad libitum smoking behavior. Psychopharmacology 140, 185–190 (1998).
Caskey, N. H., Jarvik, M. E. & Wirshing, W. C. The effects of dopaminergic D2 stimulation and blockade on smoking behavior. Exp. Clin. Psychopharm. 7, 72–78 (1999).
Caskey, N. H. et al. Modulating tobacco smoking rates by dopaminergic stimulation and blockade. Nicotine Tob. Res. 4, 259–266 (2002).
Dawe, S., Gerada, C., Russell, M. A. & Gray, J. A. Nicotine intake in smokers increases following a single dose of haloperidol. Psychopharmacology 117, 110–115 (1995).
McEvoy, J. P., Freudenreich, O., Levin, E. D. & Rose, J. E. Haloperidol increases smoking in patients with schizophrenia. Psychopharmacology 119, 124–126 (1995).
Brauer, L. H., Cramblett, M. J., Paxton, D. A. & Rose, J. E. Haloperidol reduces smoking of both nicotine-containing and denicotinized cigarettes. Psychopharmacology 159, 31–37 (2001).
Mahler, S. V. & de Wit, H. Effects of haloperidol on reactions to smoking cues in humans. Behav. Pharmacol. 16, 123–126 (2005).
Lee, C., Frangou, S., Russell, M. A. & Gray, J. A. Effect of haloperidol on nicotine-induced enhancement of vigilance in human subjects. J. Psychopharmacology 11, 253–257 (1997).
Pomerleau, C. S., Pomerleau, O. F. & Majchrzak, M. J. Mecamylamine pretreatment increases subsequent nicotine self-administration as indicated by changes in plasma nicotine level. Psychopharmacology 91, 391–393 (1987).
Stolerman, I. P., Goldfarb, T., Fink, R. & Jarvik, M. E. Influencing cigarette smoking with nicotine antagonists. Psychopharmacologia 28, 247–259 (1973).
Nemeth-Coslett, R., Henningfield, J. E., O'Keeffe, M. K. & Griffiths, R. R. Effects of mecamylamine on human cigarette smoking and subjective ratings. Psychopharmacology 88, 420–425 (1986).
Rose, J. E., Behm, F. M., Westman, E. C. & Bates, J. E. Mecamylamine acutely increases human intravenous nicotine self-administration. Pharmacol. Biochem. Behav. 76, 307–13 (2003).
Rose, J. E., Sampson, A., Levin, E. D. & Henningfield, J. E. Mecamylamine increases nicotine preference and attenuates nicotine discrimination. Pharmacol. Biochem. Behav. 32, 933–938 (1989).
Perkins, K. A. et al. Effects of central and peripheral nicotinic blockade on human nicotine discrimination. Psychopharmacology 142, 158–164 (1999).
Sacco, K. A. et al. Effects of cigarette smoking on spatial working memory and attentional deficits in schizophrenia: involvement of nicotinic receptor mechanisms. Arch. Gen. Psychiatry 62, 649–659 (2005).
Eissenberg, T., Griffiths, R. R. & Stitzer, M. L. Mecamylamine does not precipitate withdrawal in cigarette smokers. Psychopharmacology 127, 328–336 (1996).
Pickworth, W. B., Fant, R. V., Butschky, M. F. & Henningfield, J. E. Effects of mecamylamine on spontaneous EEG and performance in smokers and non-smokers. Pharmacol. Biochem. Behav. 56, 181–187 (1997).
Cousins, M. S., Stamat, H. M. & de Wit, H. Effects of a single dose of baclofen on self-reported subjective effects and tobacco smoking. Nicotine Tob. Res. 3, 123–129 (2001).
Houtsmuller, E. J., Thornton, J. A. & Stitzer, M. L. Effects of selegiline (L-deprenyl) during smoking and short-term abstinence. Psychopharmacology 163, 213–220 (2002).
LeSage, M. G. et al. Continuous nicotine infusion reduces nicotine self-administration in rats with 23-h/day access to nicotine. Pharmacol. Biochem. Behav. 72, 279–289 (2002).
Malin, D. H. et al. Nicotine alleviation of nicotine abstinence syndrome is naloxone-reversible. Pharmacol. Biochem. Behav. 53, 81–85 (1996).
Dwoskin, L. P., Crooks, P. A., Teng, L., Green, T. A. & Bardo, M. T. Acute and chronic effects of nornicotine on locomotor activity in rats: altered response to nicotine. Psychopharmacology 145, 442–451 (1999).
Gaddnas, H., Pietila, K. & Ahtee, L. Effects of chronic oral nicotine treatment and its withdrawal on locomotor activity and brain monoamines in mice. Behav. Brain Res. 113, 65–72 (2000).
Miller, D. K., Wilkins, L. H., Bardo, M. T., Crooks, P. A. & Dwoskin, L. P. Once weekly administration of nicotine produces long-lasting locomotor sensitization in rats via a nicotinic receptor-mediated mechanism. Psychopharmacology 156, 469–476 (2001).
Faraday, M. M., O'Donoghue, V. A. & Grunberg, N. E. Effects of nicotine and stress on locomotion in Sprague-Dawley and Long-Evans male and female rats. Pharmacol. Biochem. Behav. 74, 325–333 (2003).
James, J. R., Villanueva, H. F., Johnson, J. H., Arezo, S. & Rosecrans, J. A. Evidence that nicotine can acutely desensitize central nicotinic acetylcholinergic receptors. Psychopharmacology 114, 456–462 (1994).
Metzger, K. L. et al. Pharmacokinetic and behavioral characterization of a long-term antipsychotic delivery system in rodents and rabbits. Psychopharmacology 190, 201–211 (2007).
Bruijnzeel, A. W. & Markou, A. Characterization of the effects of bupropion on the reinforcing properties of nicotine and food in rats. Synapse 50, 20–28 (2003).
Rauhut, A. S., Neugebauer, N., Dwoskin, L. P. & Bardo, M. T. Effect of bupropion on nicotine self-administration in rats. Psychopharmacology 169, 1–9 (2003).
Glick, S. D., Maisonneuve, I. M. & Kitchen, B. A. Modulation of nicotine self-administration in rats by combination therapy with agents blocking α3 β4 nicotinic receptors. Eur. J. Pharmacol. 448, 185–191 (2002).
Rauhut, A. S., Dwoskin, L. P. & Bardo, M. T. Tolerance does not develop to the decrease in nicotine self-administration produced by repeated bupropion administration. Nicotine Tob. Res. 7, 901–907 (2005).
Malin, D. H. et al. Bupropion attenuates nicotine abstinence syndrome in the rat. Psychopharmacology 184, 494–503 (2006).
Cryan, J. F., Bruijnzeel, A. W., Skjei, K. L. & Markou, A. Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology 168, 347–358 (2003).
Slemmer, J. E., Martin, B. R. & Damaj, M. I. Bupropion is a nicotinic antagonist. J. Pharmacol. Exp. Ther. 295, 321–327 (2000).
Wilkinson, J. L., Palmatier, M. I. & Bevins, R. A. Preexposure to nicotine alters the subsequent locomotor stimulant effects of bupropion in rats. Nicotine Tob. Res. 8, 141–146 (2006).
Wiley, J. L., Lavecchia, K. L., Martin, B. R. & Damaj, M. I. Nicotine-like discriminative stimulus effects of bupropion in rats. Exp. Clin. Psychopharmacol. 10, 129–135 (2002).
Desai, R. I., Barber, D. J. & Terry, P. Dopaminergic and cholinergic involvement in the discriminative stimulus effects of nicotine and cocaine in rats. Psychopharmacology 167, 335–343 (2003).
Bondarev, M. L., Bondareva, T. S., Young, R. & Glennon, R. A. Behavioral and biochemical investigations of bupropion metabolites. Eur. J. Pharmacol. 474, 85–93 (2003).
Young, R. & Glennon, R. A. Nicotine and bupropion share a similar discriminative stimulus effect. Eur. J. Pharmacol. 443, 113–118 (2002).
Corrigall, W. A. & Coen, K. M. Opiate antagonists reduce cocaine but not nicotine self-administration. Psychopharmacology 104, 167–170 (1991).
DeNoble, V. J. & Mele, P. C. Intravenous nicotine self-administration in rats: effects of mecamylamine, hexamethonium and naloxone. Psychopharmacology 184, 266–272 (2006).
Corrigall, W. A. & Coen, K. M. Selective dopamine antagonists reduce nicotine self-administration. Psychopharmacology 104, 171–176 (1991).
Emerich, D. F., Norman, A. B. & Sanberg, P. R. Nicotine potentiates the behavioral effects of haloperidol. Psychopharmacol. Bull. 27, 385–390 (1991).
Emerich, D. F., Zanol, M. D., Norman, A. B., McConville, B. J. & Sanberg, P. R. Nicotine potentiates haloperidol-induced catalepsy and locomotor hypoactivity. Pharmacol. Biochem. Behav. 38, 875–880 (1991).
Brioni, J. D., Kim, D. J., O'Neill, A. B., Williams, J. E. & Decker, M. W. Clozapine attenuates the discriminative stimulus properties of (-)-nicotine. Brain Res. 643, 1–9 (1994).
Arnold, B. et al. 5HT3 receptor antagonists do not block nicotine induced hyperactivity in rats. Psychopharmacology 119, 213–221 (1995).
Kuo, D. Y. et al. Nicotine-induced hyperlocomotion is not modified by the estrous cycle, ovariectomy and estradiol replacement at physiological level. Chin. J. Physiol. 42, 83–88 (1999).
Stolerman, I. P., Kumar, R. & Reavill, C. Discriminative stimulus effects of cholinergic agonists and the actions of their antagonists. Psychopharmacol. Ser. 4, 32–43 (1988).
Watkins, S. S., Epping-Jordan, M. P., Koob, G. F. & Markou, A. Blockade of nicotine self-administration with nicotinic antagonists in rats. Pharmacol. Biochem. Behav. 62, 743–751 (1999).
Glick, S. D., Visker, K. E. & Maisonneuve, I. M. An oral self-administration model of nicotine preference in rats: effects of mecamylamine. Psychopharmacology 128, 426–431 (1996).
Mansbach, R. S., Chambers, L. K. & Rovetti, C. C. Effects of the competitive nicotinic antagonist erysodine on behavior occasioned or maintained by nicotine: comparison with mecamylamine. Psychopharmacology 148, 234–242 (2000).
Rauhut, A. S., Mullins, S. N., Dwoskin, L. P. & Bardo, M. T. Reboxetine: attenuation of intravenous nicotine self-administration in rats. J. Pharmacol. Exp. Ther. 303, 664–672 (2002).
Liu, X. et al. Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology 32, 710–718 (2007).
Olausson, P., Jentsch, J. D. & Taylor, J. R. Nicotine enhances responding with conditioned reinforcement. Psychopharmacology 171, 173–178 (2004).
Grabus, S. D., Martin, B. R., Brown, S. E. & Damaj, M. I. Nicotine place preference in the mouse: influences of prior handling, dose and strain and attenuation by nicotinic receptor antagonists. Psychopharmacology 184, 456–463 (2006).
Iwamoto, E. T. Nicotine conditions place preferences after intracerebral administration in rats. Psychopharmacology 100, 251–257 (1990).
Fudala, P. J., Teoh, K. W. & Iwamoto, E. T. Pharmacologic characterization of nicotine-induced conditioned place preference. Pharmacol. Biochem. Behav. 22, 237–241 (1985).
Sahraei, H. et al. The effects of nitric oxide on the acquisition and expression of nicotine-induced conditioned place preference in mice. Eur. J. Pharmacol. 503, 81–87 (2004).
Gould, T. J., Rukstalis, M. & Lewis, M. C. Atomoxetine and nicotine enhance prepulse inhibition of acoustic startle in C57BL/6 mice. Neurosci. Lett. 377, 85–90 (2005).
Fattore, L., Cossu, G., Martellotta, M. C. & Fratta, W. Baclofen antagonizes intravenous self-administration of nicotine in mice and rats. Alcohol Alcohol. 37, 495–498 (2002).
Paterson, N. E., Froestl, W. & Markou, A. The GABAB receptor agonists baclofen and CGP44532 decreased nicotine self-administration in the rat. Psychopharmacology 172, 179–186 (2004).
Palmatier, M. I. & Bevins, R. A. Examination of GABAergic and dopaminergic compounds in the acquisition of nicotine-conditioned hyperactivity in rats. Neuropsychobiology 45, 87–94 (2002).
Rollema, H. et al. Pharmacological profile of the α(4)β(2) nicotinic acetylcholine receptor partial agonist varenicline, an effective smoking cessation aid. Neuropharmacology 52, 985–994 (2007).
Cohen, C., Perrault, G., Voltz, C., Steinberg, R. & Soubrie, P. SR141716, a central cannabinoid (CB(1)) receptor antagonist, blocks the motivational and dopamine-releasing effects of nicotine in rats. Behav. Pharmacol. 13, 451–463 (2002).
Cohen, C., Perrault, G., Griebel, G. & Soubrie, P. Nicotine-associated cues maintain nicotine-seeking behavior in rats several weeks after nicotine withdrawal: reversal by the cannabinoid (CB1) receptor antagonist, rimonabant (SR141716). Neuropsychopharmacology 30, 145–155 (2005).
De Vries, T. J., de Vries, W., Janssen, M. C. & Schoffelmeer, A. N. Suppression of conditioned nicotine and sucrose seeking by the cannabinoid-1 receptor antagonist SR141716A. Behav. Brain Res. 161, 164–168 (2005).
Forget, B., Hamon, M. & Thiebot, M. H. Cannabinoid CB1 receptors are involved in motivational effects of nicotine in rats. Psychopharmacology 181, 722–734 (2005).
Zaniewska, M., McCreary, A. C., Przegaliski, E. & Filip, M. Evaluation of the role of nicotinic acetylcholine receptor subtypes and cannabinoid system in the discriminative stimulus effects of nicotine in rats. Eur. J. Pharmacol. 540, 96–106 (2006).
Paterson, N. E. & Markou, A. Increased GABA neurotransmission via administration of γ-vinyl GABA decreased nicotine self-administration in the rat. Synapse 44, 252–253 (2002).
Dewey, S. L. et al. A pharmacologic strategy for the treatment of nicotine addiction. Synapse 31, 76–86 (1999).
Bevins, R. A., Besheer, J. & Pickett, K. S. Nicotine-conditioned locomotor activity in rats: dopaminergic and GABAergic influences on conditioned expression. Pharmacol. Biochem. Behav. 68, 135–145 (2001).
Glick, S. D., Maisonneuve, I. M., Dickinson, H. A. & Kitchen, B. A. Comparative effects of dextromethorphan and dextrorphan on morphine, methamphetamine, and nicotine self-administration in rats. Eur. J. Pharmacol. 422, 87–90 (2001).
Wright, M. J., Vann, R. E., Gamage, T. F., Damaj, M. I. & Wiley, J. L. Comparative effects of dextromethorphan and dextrorphan on nicotine discrimination in rats. Pharmacol. Biochem. Behav. 85, 507–513 (2006).
Zakharova, E. S., Danysz, W. & Bespalov, A. Y. Drug discrimination analysis of NMDA receptor channel blockers as nicotinic receptor antagonists in rats. Psychopharmacology 179, 128–135 (2005).
Sannerud, C. A., Prada, J., Goldberg, D. M. & Goldberg, S. R. The effects of sertraline on nicotine self-administration and food-maintained responding in squirrel monkeys. Eur. J. Pharmacol. 271, 461–469 (1994).
Semenova, S. & Markou, A. Clozapine treatment attenuated somatic and affective signs of nicotine and amphetamine withdrawal in subsets of rats exhibiting hyposensitivity to the initial effects of clozapine. Biol. Psychiatry 54, 1249–1264 (2003).
Levin, E. D., Petro, A. & Caldwell, D. P. Nicotine and clozapine actions on pre-pulse inhibition deficits caused by N-methyl-D-aspartate (NMDA) glutamatergic receptor blockade. Prog. Neuropsychopharmaco. Biol. Psychiatry 29, 581–586 (2005).
Harrison, A. A., Liem, Y. T. & Markou, A. Fluoxetine combined with a serotonin-1A receptor antagonist reversed reward deficits observed during nicotine and amphetamine withdrawal in rats. Neuropsychopharmacology 25, 55–71 (2001).
Acknowledgements
The authors are grateful to A. Chausmer of the Division of Basic Neuroscience and Behavioural Research at the National Institute on Drug Abuse for helping to organize us as a group, and for reading and commenting on the manuscript. We also thank P. Olausson, R. Ray, M. Sofuoglu and A. Strasser for their helpful input, and M. Foster for her assistance with the manuscript preparation. This work was supported in part by Transdisciplinary Tobacco Use Research Center grants from the National Cancer Institute, the National Institute on Drug Abuse, and National Institute on Alcohol Abuse and Addiction (P50CA84718 to C.L.; P50AA15632 to S.O.M.; P50DA013333 to D. Hatsukami, which partially supports M.G.L.), and by other grants (KO5AA014715 to S.O.M.; R01DA020136 to M.G.L.).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
C.L. has served as a consultant/advisor to Astra Zeneca, GlaxoSmithKline, Pfizer and Cephalon regarding medications for nicotine dependence.
M.L. has conducted research that has been funded by GlaxoSmithKline.
K.P. has previously consulted with GlaxoSmithKline, Elan and Pfizer.
S.O'M. has received research support, principally clinical supplies or contracts as a study site, from Alkermes, Bristol–Myers Squibb, Lipha–Merck, GlaxoSmithKline, Pfizer, Ortho–McNeil and Sanofi–Aventis, and has served as a consultant for Eli Lilly, Ortho–McNeil, GlaxoSmithKline, She is an inventor on patents held by Yale University for naltrexone for smoking cessation.
S.S. has served as a consultant to NuPathe developing drug delivery technologies and has conducted research related to nicotine receptor pharmacology that has been funded by AstraZeneca.
N.B. has served as a consultant to several pharmaceutical companies that market smoking cessation medications, specifically Pfizer and GlaxoSmithKline.
W.C. is the sole proprietor of a science consulting business in which a current contract with the National Institute on Drug Abuse includes activities related to the Transdisciplinary Tobacco Use Research Centers and the National Cooperative Drug Discovery Groups (in the case of the latter, he has reseach discussions with the pharmaceutical industry partners involved in the initiative, but not for additional remuneration). In addition, he has served as a consultant/advisor to, and/or conducted research funded by Cantab, GlaxoSmithKline, Pfizer and Pharmacia.
Glossary
- Partial agonist
-
A full agonist produces the system maximal response, whereas a partial agonist produces a maximal response that is below that of the system maximum (and that of a full agonist). As well as producing a submaximal response, partial agonists antagonize full agonists.
- Face-valid model
-
A model that looks or appears to be a valid representation of what it purports to measure.
- Translational research
-
The term is generally used to describe research efforts intended to apply advances in basic science to the clinical research setting. For drug discovery and development, the term refers to research intended to progress basic science discoveries into medications.
- Sensitization
-
The phenomenon in which the biological or behavioural response to a drug increases with successive exposures to it. Sensitization may be elicited by repeated drug treatment itself or by coupling the drug with a particular environment.
- CREB
-
This is an acronym for cyclic AMP response element binding and refers to proteins that increase or decrease the transcription of certain genes. CREB has been associated with many functions, including the formation of long-term memories, neuronal survival and cancers.
- ΔFosB
-
Like CREB, this is also a transcription factor. It has been found to accumulate in neurons in the synaptic field of dopamine neurons following exposure to different dependence-producing drugs, and persists for protracted periods of time, suggesting that it may be important in sustaining changes in gene expression.
- Intracranial self-stimulation
-
Also called brain-stimulation reward, this refers to the phenomenon in which animals will perform an operant task to receive an electrical current delivered through electrodes implanted in certain parts of their brains, such as the lateral hypothalamus.
- Stroop colour-word test
-
This task measures how well an individual resists interference between incongruent stimuli. In the standard test, words can be congruent (for example, the colour green presented in green text) or incongruent (for example, the colour green presented in blue text). This task has been adapted to measure interference from words relating to drugs (for example, ashtray) or mood-related words (for example, sad).
- Event-related potentials
-
Event-related potentials are positive and negative electroencephalogram voltage deflections in response to specific stimuli, for example, visual, auditory or somatosensory.
- Five-choice serial reaction time task
-
A task used in animal behavioural pharmacological studies that approximates continuous performance tests in humans. Subjects are required to track a light stimulus at the rear of one of five holes in a response board. Nose poke behaviour in the correct hole produces food reward in a separate food hopper. Errors of commission, omission and premature responding are punished with a form of task reset.
- Continuous performance task
-
This task measures sustained attention and vigilance. Subjects view a series of target (requiring a response) and non-target (not requiring a response) stimuli, such as letters, numbers or figures.
- Fear-conditioning models
-
In these approaches, a neutral stimulus that is paired repeatedly with an aversive stimulus can elicit a fear response measurable in animals as physiological (for example, heart rate) and behavioural (for example, immobility) changes, and in humans with self-report and galvanic skin response.
- Startle response
-
A physiological response, such as eye blink or bulk muscle contraction, to the presentation of an unexpected, brief stimulus, typically a quantifiable one such as a flash of light or audible tone.
Rights and permissions
About this article
Cite this article
Lerman, C., LeSage, M., Perkins, K. et al. Translational research in medication development for nicotine dependence. Nat Rev Drug Discov 6, 746–762 (2007). https://doi.org/10.1038/nrd2361
Issue Date:
DOI: https://doi.org/10.1038/nrd2361
This article is cited by
-
Individual variations in motives for nicotine self-administration in male rats: evidence in support for a precision psychopharmacology
Translational Psychiatry (2024)
-
Liraglutide attenuates nicotine self-administration as well as nicotine seeking and hyperphagia during withdrawal in male and female rats
Psychopharmacology (2023)
-
12-h abstinence-induced functional connectivity density changes and craving in young smokers: a resting-state study
Brain Imaging and Behavior (2019)
-
Initial Cross-Over Test of A Positive Allosteric Modulator of Alpha-7 Nicotinic Receptors to Aid Cessation in Smokers With Or Without Schizophrenia
Neuropsychopharmacology (2018)
-
The effects of cannabidiol on impulsivity and memory during abstinence in cigarette dependent smokers
Scientific Reports (2018)