Abstract
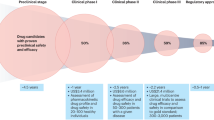
Experimental medicine is the use of innovative measurements, models and designs in studying human subjects for establishing proof of mechanism and concept of new drugs, for exploring the potential for market differentiation for successful drug candidates, and for efficiently terminating the development of unsuccessful ones. Humans are the ultimate 'model' because of the uncertain validity and efficacy of novel targets and drug candidates that emerge from genomics, combinatorial chemistry and high-throughput screening and the use of poorly predictive preclinical models. The in-depth investigation of the effects of drugs and the nature of disease progression is becoming ever more feasible because of advances in clinical biomarkers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Sheiner, L. B. Learning versus confirming in clinical drug development. Clin. Pharmacol. Ther. 61, 275–291 (1997).
Levy, G. in Pharmacodynamics and Toxicokinetics in Rational Drug Development (Plenum, New York, 1993).
Booz Allen Hamilton & Credit Suisse First Boston. Know thy R&D enemy: the key to fighting attrition. IN VIVO 19, 59 (2005).
Meyer, J. M. & Ginsburg, G. S. The path to personalized medicine. Curr. Opin. Chem. Biol. 6, 434–438 (2002).
Pharma 2010: The Threshold on Innovation. IBM Business Consulting Services [online], <http://www-1.ibm.com/services/us/index.wss/xs/imc/a1001099> (April 2004).
Branca, M. A. Impact of technological advances on pharmaceutical productivity. SPECTRUM Pharmaceutical Industry Dynamics [online],(May 2004).
Lehman Brothers. The Fruits of Genomics [online], <http://www.lehman.com> (2001).
Winslow, R. What makes a drug too risky? there's no easy answer. Wall Street J. (11 Feb 2005) [online], <http://www.aegis.com/news/wsj/2005/WJ050206.html>
Greaves, P., Williams, A. & Eve, M. First dose of potential new medicines to humans: how animals help. Nature Rev. Drug Discov. 3, 226–236 (2004).
Ratner, M. L. How experimental medicine is affecting big pharma. IN VIVO 18, 59 (2004)
Kola, I. & Landis, J. Can the pharmaceutical industry reduce attrition rates? Nature Rev. Drug Discov. 3, 711–715 (2004).
Leaf, C. Why we're losing the war on cancer — and how to win it. Fortune 9, March (2004).
Polmar, S., Diaz-Gonzalez, F., Dougados, M., Ortiz, P. & de-Miguel, G. Limited clinical efficacy of a leukotriene B4 receptor (LTB4) antagonist in patients with active rheumatoid arthritis (RA). Arthritis Rheum. 50, S239 (2004).
Tarazi F. I., Zhang K. & Baldessarini R. J. Review: dopamine D4 receptors: beyond schizophrenia. J. Recept. Signal Transduct. Res. 24, 131–147 (2004).
Ikonomidou, C. & Turski, L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 6, 383–486 (2002).
DiMasi, J. A. The value of improving the productivity of the drug development process. Pharmacoeconomics 20 (Suppl. 3), 1–10 (2002).
BoozAllen Hamilton. Pharmaceutical Industry — 2002 Year in Review (2003).
Altshuler, J., Flanagan A., Guy P., Steiner, M. & Tollman, P. Boston Consulting Group. A Revolution in R&D – The Impact of Genomics [online], <http://www.bcg.com/publications/publications_search_results.jsp?PUBID=653> (November 26 2001).
Roner, L. Bridging the great divide between R&D and Commercial. [online], <http://www.eyeforpharma.com/index.asp?news=45028> (14 Feb 2005).
Gray, M. L. et al. Toward imaging biomarkers for osteoarthritis. Clin. Orthop. Relat. Res. 427 (Suppl.), S175–S181 (2004).
Lohmander, L. S. Markers of altered metabolism in osteoarthritis. J. Rheumatol. Suppl. 70, 28–35 (2004)
Graichen, H., von Eisenhart-Rothe, R., Vogl, T., Englmeier, K. H. & Eckstein, F. Quantitative assessment of cartilage status in osteoarthritis by quantitative magnetic resonance imaging: technical validation for use in analysis of cartilage volume and further morphologic parameters. Arthritis Rheum. 50, 811–816 (2004).
Tamez-Pena, J. G., Barbu-McInnis, M. & Totterman, S. Knee cartilage extraction and bone-cartilage interface analysis from 3D MRI data sets. Proc. SPIE 5370, 1774–1784 (2004).
Tiderius, C. J., Olsson, L. E., Leander, P., Ekberg, O. & Dahlberg, L. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) in early knee osteoarthritis. Magn Reson Med. 49, 488–492 (2003).
Williams, A. et al. Glycosaminoglycan distribution in cartilage as determined by delayed gadolinium-enhanced MRI of cartilage (dGEMRIC): potential clinical applications. AJR Am. J. Roentgenol. 182, 167–172 (2004).
Branca, M. A new perspective on pharmacogenomics in drug development. SPECTRUM Therapy Markets and Emerging Technologies [online], (June 2004).
Drews, J. Strategic trends in the drug industry. Drug Discov. Today 8, 411–420 (2003).
Pickering, L. Systems biology approaches in drug discovery. SPECTRUM Drug Discovery and Design, 2610–2618 [online], (Nov 2003).
Kaiser, J. NCI gears up for Cancer Genome Project. Science 307, 1182 (2005)
Echt, D. S. et al. Mortality and morbidity in patients receiving encainide, flecainide or placebo. The Cardiac Arrhythmia Suppression Trial. N. Engl. J. Med. 324, 781–788 (1991)
Fleming, T. R. & DeMets, D. L. Surrogate endpoints in clinical trials: are we being misled? Ann. Intern. Med. 125, 605–613 (1996).
US FDA. Challenge and Opportunity on the Critical Path to New Medical Products [online], <http://www.fda.gov/oc/initiatives/criticalpath/> (2004).
Lesko, L. J. & Atkinson Jr., A. J. Use of biomarkers and surrogate endpoints in drug development and regulatory decision making: criteria, validation, strategies. Annu. Rev. Pharmacol. Toxicol. 41, 347–661 (2001).
Katz, R. Biomarkers and surrogate markers: an FDA perspective. NeuroRx 1, 189–195 (2004).
Prentice, R. L. Surrogate endpoints in clinical trials: definition and operational criteria. Stat. Med. 8, 431–440 (1989).
Fischman, A. J. et al. Positron emission tomographic analysis of central 5-hydroxytryptamine2 receptor occupancy in healthy volunteers treated with the novel antipsychotic agent, ziprasidone. J. Pharmacol. Exp. Ther. 279, 939–947 (1996).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
B.H.L and S.A.W. are employees of and equity holders in Pfizer Inc., which has a strategic alliance with Virtualscopics LLC.
Related links
Related links
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Littman, B., Williams, S. The ultimate model organism: progress in experimental medicine. Nat Rev Drug Discov 4, 631–638 (2005). https://doi.org/10.1038/nrd1800
Issue Date:
DOI: https://doi.org/10.1038/nrd1800
This article is cited by
-
Droplet microarray platforms for high-throughput drug screening
Microchimica Acta (2023)
-
Altered brainstem responses to modafinil in schizophrenia: implications for adjunctive treatment of cognition
Translational Psychiatry (2018)
-
A Review of the Institute of Medicine’s Analysis of using Chimpanzees in Biomedical Research
Science and Engineering Ethics (2014)
-
The Nuremberg Code subverts human health and safety by requiring animal modeling
BMC Medical Ethics (2012)
-
Create a translational medicine knowledge repository - Research downsizing, mergers and increased outsourcing have reduced the depth of in-house translational medicine expertise and institutional memory at many pharmaceutical and biotech companies: how will they avoid relearning old lessons?
Journal of Translational Medicine (2011)