Key Points
-
The concept of a 'target-rich, lead-poor' pipeline in drug discovery, and widespread concern about the attrition rate of chemical compounds in (pre)clinical development, are together fuelling the search for better quality hits and chemical lead series.
-
A particular approach to lead identification for drug discovery, which offers a number of attractive features compared with high-throughput screening, involves the selection, screening and optimization of 'fragments'. Fragments typically have Mr = 120–250 and binding affinities in the range mM–30 μM. However, the weak absolute potency of fragments belies their high efficiency as ligands, because fragments are extremely potent for their size.
-
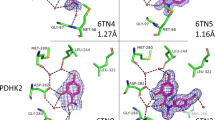
Generally, fragments are identified using a biophysical screening method, most commonly NMR or protein crystallography supported by a conventional enzyme bioassay. Consequently, information about the structure of the fragment–protein binding interaction is generated as part of the screening.
-
This structural information means that it is possible to incorporate a large element of design in optimizing the fragment into a high-affinity lead, either by growing additional binding groups or joining two fragments together. As a result, fragments can be optimized into nanomolar leads via the synthesis of significantly fewer compounds than in traditional approaches.
-
Furthermore, starting the chemical optimization stage with a low-molecular-mass fragment is likely to produce leads whose Mr is still within the range desired for lead-likeness.
-
This review discusses fragment-based lead discovery, and focuses on the output of this new approach by collating published examples from 25 protein targets. These targets are primarily enzymes and the screening techniques used include X-ray crystallography, nuclear magnetic resonance (NMR) spectroscopy, in vitro bioassays and mass spectrometry.
Abstract
Fragment-based lead discovery is gaining momentum in both large pharmaceutical companies and biotechnology laboratories as a complementary approach to traditional screening. This is because fragment-based approaches require significantly fewer compounds to be screened and synthesized, and are showing a high success rate in generating chemical series with lead-like properties. Compared with traditional screening hits, the starting fragments have considerably lower molecular mass, and although the binding interactions of these fragments with a target protein are weak, they are structurally understood through X-ray crystallography or NMR, and they exhibit high 'ligand efficiency'. Here, we use examples from 25 different protein targets to describe chemical strategies that exploit this structural knowledge to rapidly develop fragments into high-affinity leads.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Boehm, H. J. et al. Novel inhibitors of DNA gyrase: 3D structure based biased needle screening, hit validation by biophysical methods, and 3D guided optimization. A promising alternative to random screening. J. Med. Chem. 43, 2664–2674 (2000). An early description of a strategy to identify needles via virtual screening, in vitro screening and validation by biophysical methods; also provides an example of fragment evolution applied to DNA gyrase.
Fejzo, J. et al. The SHAPES strategy: an NMR-based approach for lead generation in drug discovery. Chem. Biol. 6, 755–769 (1999).
Maly, D. J., Choong, I. C. & Ellman, J. A. Combinatorial target-guided ligand assembly: identification of potent subtype-selective c-Src inhibitors. Proc. Natl Acad. Sci. USA 97, 2419–2424 (2000).
Liebeschuetz, J. W. et al. PRO_SELECT: combining structure-based drug design and array-based chemistry for rapid lead discovery. 2. The development of a series of highly potent and selective factor Xa inhibitors. J. Med. Chem. 45, 1221–1232 (2002).
Jencks, W. P. On the attribution and additivity of binding energies. Proc. Natl Acad. Sci. USA 78, 4046–4050 (1981).
Farmer, P. S. & Ariens, E. J. Speculations on the design of nonpeptidic peptidomimetics. Trends Pharmacol. Sci. 3, 362–365 (1982).
Shuker, S. B., Hajduk, P. J., Meadows, R. P. & Fesik, S. W. Discovering high-affinity ligands for proteins: SAR by NMR. Science 274, 1531–1534 (1996). Prototype description of the SAR by NMR technique and an early example of fragment linking to obtain nM ligands for the FK506-binding protein.
Pellecchia, M., Sem, D. S. & Wuthrich, K. NMR in drug discovery. Nature Rev. Drug Discov. 1, 211–219 (2002).
Kuhn, P., Wilson, K., Patch, M. G. & Stevens, R. C. The genesis of high-throughput structure-based drug discovery using protein crystallography. Curr. Opin. Chem. Biol. 6, 704–710 (2002).
Blundell, T. L., Jhoti, H. & Abell, C. High-throughput crystallography for lead discovery in drug design. Nature Rev. Drug Discov. 1, 45–54 (2002). Review of the techniques required for high-throughput X-ray crystallography and discussion of this as a screening technique for lead discovery.
Hann, M. M., Leach, A. R. & Harper, G. Molecular complexity and its impact on the probability of finding leads for drug discovery. J. Chem. Inf. Comput. Sci. 41, 856–864 (2001).
Oprea, T. I., Davis, A. M., Teague, S. J. & Leeson, P. D. Is there a difference between leads and drugs? A historical perspective. J. Chem. Inf. Comput. Sci. 41, 1308–1315 (2001).
Teague, S. J., Davis, A. M., Leeson, P. D. & Oprea, T. The design of leadlike combinatorial libraries. Angew. Chem. Int. Ed. Engl. 38, 3743–3748 (1999).
Wenlock, M. C., Austin, R. P., Barton, P., Davis, A. M. & Leeson, P. D. A comparison of physiochemical property profiles of development and marketed oral drugs. J. Med. Chem. 46, 1250–1256 (2003).
Vieth, M. et al. Characteristic physical properties and structural fragments of marketed oral drugs. J. Med. Chem. 47, 224–232 (2004).
Lipinski, C. A., Lombardo, F., Dominy, B. W. & Feeney, P. J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 46, 3–26 (2001).
Veber, D. F. et al. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 45, 2615–2623 (2002).
Congreve, M., Carr, R., Murray, C. & Jhoti, H. A 'rule of three' for fragment-based lead discovery? Drug Discov. Today 8, 876–877 (2003).
Erlanson, D. A. et al. Site-directed ligand discovery. Proc. Natl Acad. Sci. USA 97, 9367–9372 (2000).
Frederickson, M., Gill, A. L., Padova, A. & Congreve, M. S. Preparation of indoles as p38 MAP kinase inhibitors. (Astex Technology Limited, UK. UK Patent WO 03/087087 (2003).
Wendt, M. D. et al. Identification of novel binding interactions in the development of potent, selective 2-naphthamidine inhibitors of urokinase: synthesis, structural analysis, and sar of N-phenyl amide 6-substitution. J. Med. Chem. 47, 303–324 (2004).
Bohm, H. J., Banner, D. W. & Weber, L. Combinatorial docking and combinatorial chemistry: design of potent non-peptide thrombin inhibitors. J. Comput. Aided Mol. Des. 13, 51–56 (1999).
van Dongen, M. J. P. et al. Structure-based screening as applied to human FABP4: a highly efficient alternative to HTS for hit generation. J. Am. Chem. Soc. 124, 11874–11880 (2002).
Hajduk, P. J. et al. Novel inhibitors of Erm methyltransferases from NMR and parallel synthesis. J. Med. Chem. 42, 3852–3859 (1999).
Murray, C. W. & Verdonk, M. L. The consequences of translational and rotational entropy lost by small molecules on binding to proteins. J. Comput. Aided Mol. Des. 16, 741–753 (2002).
Green, N. M. Avidin. Adv. Protein Chem. 29, 85–133 (1975).
Rao, J. & Whitesides, G. M. Tight binding of a dimeric derivative of vancomycin with dimeric L-Lys-D-Ala-D-Ala. J. Am. Chem. Soc. 119, 10286–10290 (1997).
Rao, J., Lahiri, J., Weis, R. M. & Whitesides, G. M. Design, synthesis, and characterization of a high-affinity trivalent system derived from vancomycin and L-Lys-D-Ala-D-Ala. J. Am. Chem. Soc. 122, 2698–2710 (2000).
Pang, Y. P., Quiram, P., Jelacic, T., Hong, F. & Brimijoin, S. Highly potent, selective, and low cost bis-tetrahydroaminacrine inhibitors of acetylcholinesterase. Steps toward novel drugs for treating Alzheimer's disease. J. Biol. Chem. 271, 23646–23649 (1996).
Pereira, P. J. et al. Human β-tryptase is a ring-like tetramer with active sites facing a central pore. Nature 392, 306–311 (1998).
Burgess, L. E. et al. Potent selective nonpeptidic inhibitors of human lung tryptase. Proc. Natl Acad. Sci. USA 96, 8348–8352 (1999).
Rice, K. D. et al. Dibasic inhibitors of human mast cell tryptase. Part 2: structure–activity relationships and requirements for potent activity. Bioorg. Med. Chem. Lett. 10, 2361–2366 (2000).
Hajduk, P. J. et al. Discovery of potent nonpeptide inhibitors of stromelysin using SAR by NMR. J. Am. Chem. Soc. 119, 5818–5827 (1997).
Erlanson, D. A. et al. In situ assembly of enzyme inhibitors using extended tethering. Nature Biotechnol. 21, 308–314 (2003).
Szczepankiewicz, B. G. et al. Discovery of a potent, selective protein tyrosine phosphatase 1B inhibitor using a linked-fragment strategy. J. Am. Chem. Soc. 125, 4087–4096 (2003).
Liu, G. et al. Fragment screening and assembly: a highly efficient approach to a selective and cell active protein tyrosine phosphatase 1B inhibitor. J. Med. Chem. 46, 4232–4235 (2003).
Swayze, E. E. et al. SAR by MS: a ligand based technique for drug lead discovery against structured RNA targets. J. Med. Chem. 45, 3816–3819 (2002).
Braisted, A. C. et al. Discovery of a potent small molecule IL-2 inhibitor through fragment assembly. J. Am. Chem. Soc. 125, 3714–3715 (2003).
Ramstrom, O. & Lehn, J. M. Drug discovery by dynamic combinatorial libraries. Nature Rev. Drug Discov. 1, 26–36 (2002).
Huc, I. & Lehn, J. M. Virtual combinatorial libraries: dynamic generation of molecular and supramolecular diversity by self-assembly. Proc. Natl Acad. Sci. USA 94, 2106–2110 (1997).
Hochgurtel, M. et al. Ketones as building blocks for dynamic combinatorial libraries: highly active neuraminidase inhibitors generated via selection pressure of the biological target. J. Med. Chem. 46, 356–358 (2003).
Hochgurtel, M. et al. Target-induced formation of neuraminidase inhibitors from in vitro virtual combinatorial libraries. Proc. Natl Acad. Sci. USA 99, 3382–3387 (2002).
Congreve, M. S. et al. Detection of ligands from a dynamic combinatorial library by X-ray crystallography. Angew. Chem. Int. Ed. Engl. 42, 4479–4482 (2003).
Bourne, Y. et al. Freeze-frame inhibitor captures acetylcholinesterase in a unique conformation. Proc. Natl Acad. Sci. USA 101, 1449–1454 (2004).
Lewis, W. G. et al. Click chemistry in situ: acetylcholinesterase as a reaction vessel for the selective assembly of a femtomolar inhibitor from an array of building blocks. Angew. Chem. Int. Ed. Engl. 41, 1053–1057 (2002).
Hajduk, P. J. et al. Identification of novel inhibitors of urokinase via NMR-based screening. J. Med. Chem. 43, 3862–3866 (2000).
Nienaber, V. L. et al. Discovering novel ligands for macromolecules using X-ray crystallographic screening. Nature Biotechnol. 18, 1105–1108 (2000). An early example of X-ray crystallography-driven screening applied to the discovery of urokinase inhibitors by fragment optimization.
Lesuisse, D. et al. SAR and X-ray. A new approach combining fragment-based screening and rational drug design: application to the discovery of nanomolar inhibitors of Src SH2. J. Med. Chem. 45, 2379–2387 (2002).
Lange, G. et al. Requirements for specific binding of low affinity inhibitor fragments to the SH2 domain of (pp60)Src are identical to those for high affinity binding of full length inhibitors. J. Med. Chem. 46, 5184–5195 (2003).
Hajduk, P. J. et al. Design of adenosine kinase inhibitors from the NMR-based screening of fragments. J. Med. Chem. 43, 4781–4786 (2000).
Hopkins, A. L, Groom, C. R & Alex, A. Ligand efficiency: a useful metric for lead selection. Drug Discov. Today 9, 430–431 (2004). This short article describes a simple but useful way to quantify 'ligand efficiency' (the contribution of each non-hydrogen atom in a lead to the overall binding affinity).
Erlanson, D. A., McDowell, R. S. & O'Brien, T. Fragment-based drug discovery. J. Med. Chem. 47, 3463–3482 (2004).
Teague, S. J. Implications of protein flexibility for drug discovery. Nature Rev. Drug Discov. 2, 527–541 (2003).
Acknowledgements
H. Jhoti for scientific input, R. Taylor for figure 1 and H. Sore for proof reading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors are employed by Astex-Technology which uses fragment-based lead discovery.
Related links
Glossary
- RULE OF FIVE
-
Identifies several key properties that should be considered for compounds with oral delivery in mind. These properties are molecular mass <500 Da, cLogP <5, number of hydrogen-bond donors ≤5 and number of hydrogen-bond acceptors ≤10.
- SURFACE PLASMON RESONANCE
-
(SPR). A phenomenon which occurs when light is reflected off thin metal films to which target molecules are immobilized and addressed by ligands in a mobile phase. If binding occurs to the immobilized target then the local refractive index changes, which leads to the apparent rate constants for the association and dissociation phases of the reaction. The ratio of these values gives the apparent equilibrium constant (affinity).
- NUCLEAR OVERHAUSER EFFECTS
-
(NOEs). Changes in the intensity of NMR signals, which are caused by through-space dipole–dipole coupling. Upper distance constraints obtained from 1H–1H NOEs are used for NMR structure determination of biological macromolecules.
- VANCOMYCIN
-
Vancomycin is an antibiotic that acts by binding to cell-wall precursors that terminate in the sequence D-Ala-D-Ala, thereby inhibiting cell-wall synthesis.
- P1′ SITE
-
The substrate residue that occurs immediately after the scissile amide bond in a protease. It is the key specificity site of matrix metalloproteases like stromelysin.
- S1 POCKET
-
The pocket on a protease occupied by the substrate residue which immediately precedes the scissile amide bond. It is the key specificity pocket of trypsin-like serine proteases such as factor Xa, urokinase, tryptase and thrombin, in which lysine or arginine are the favoured substrate residues.
Rights and permissions
About this article
Cite this article
Rees, D., Congreve,, M., Murray, C. et al. Fragment-based lead discovery. Nat Rev Drug Discov 3, 660–672 (2004). https://doi.org/10.1038/nrd1467
Issue Date:
DOI: https://doi.org/10.1038/nrd1467
This article is cited by
-
Fragment library screening by X-ray crystallography and binding site analysis on thioredoxin glutathione reductase of Schistosoma mansoni
Scientific Reports (2024)
-
Protein-templated ligand discovery via the selection of DNA-encoded dynamic libraries
Nature Chemistry (2024)
-
Synthesis of a 13C-methylene-labeled isoleucine precursor as a useful tool for studying protein side-chain interactions and dynamics
Journal of Biomolecular NMR (2024)
-
Two nucleophiles take turns
Nature Synthesis (2023)
-
Exploring protein hotspots by optimized fragment pharmacophores
Nature Communications (2021)