Key Points
-
Invasive fungal infections are a substantial medical concern, particularly in immunocompromised patients. As medical advances increase the size of this population, invasive fungal infections will continue to be a large medical issue.
-
Progress has been made recently in developing antifungal agents, but more agents, particularly those with a broad spectrum and low toxicity, are needed. New formulations of existing antifungals are being developed to address these issues.
-
Novel antifungal pathways and targets are being used to identify potential antifungal agents with new mechanisms of action, which could lead to the development of new drugs.
-
Alternative strategies to target drugs to infection sites, to repurpose agents for antifungal use and to use the immune system as adjunctive therapies are discussed in this Review.
Abstract
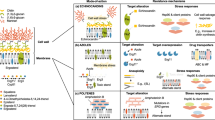
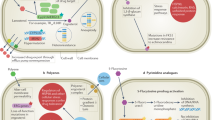
Invasive fungal infections continue to appear in record numbers as the immunocompromised population of the world increases, owing partially to the increased number of individuals who are infected with HIV and partially to the successful treatment of serious underlying diseases. The effectiveness of current antifungal therapies — polyenes, flucytosine, azoles and echinocandins (as monotherapies or in combinations for prophylaxis, or as empiric, pre-emptive or specific therapies) — in the management of these infections has plateaued. Although these drugs are clinically useful, they have several limitations, such as off-target toxicity, and drug-resistant fungi are now emerging. New antifungals are therefore needed. In this Review, I discuss the robust and dynamic antifungal pipeline, including results from preclinical academic efforts through to pharmaceutical industry products, and describe the targets, strategies, compounds and potential outcomes.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Brown, G. D., Denning, D. W. & Levitz, S. M. Tackling human fungal infections. Science 336, 647 (2012). This work reviews the current magnitude of issues relating to invasive fungal infections.
Park, B. J. et al. Estimation of the current global burden of cryptococcal meningitis among persons living with HIV/AIDS. AIDS 23, 525–530 (2009).
Patterson, T. F. et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 63, e1–e60 (2016).
Pappas, P. G. et al. Clinical practice guideline for the management of candidiasis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 62, e1–e50 (2016).
Galgiani, J. N. et al. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin. Infect. Dis. 63, e112–e146 (2016).
Perfect, J. R. et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 50, 291–322 (2010).
Kauffman, C. A., Hajjeh, R. & Chapman, S. W. Practice guidelines for the management of patients with sporotrichosis. For the Mycoses Study Group. Infectious Diseases Society of America. Clin. Infect. Dis. 30, 684–687 (2000).
Oliver, J. D. et al. F901318 represents a novel class of antifungal drug that inhibits dihydroorotate dehydrogenase. Proc. Natl Acad. Sci. USA 113, 12809–12814 (2016).
Shahid, S. K. Newer patents in antimycotic therapy. Pharm. Pat. Anal. 5, 115–134 (2016).
Pouliot, M. & Jeanmart, S. Pan assay interference compounds (PAINS) and other promiscuous compounds in antifungal research. J. Med. Chem. 59, 497–503 (2016).
Pitman, S. K., Drew, R. H. & Perfect, J. R. Addressing current medical needs in invasive fungal infection prevention and treatment with new antifungal agents, strategies and formulations. Expert Opin. Emerg. Drugs 16, 559–586 (2011). This study is an extensive review of the available antifungal agents and discusses how they are optimally used today.
Cuenca-Estrella, M. Antifungal drug resistance mechanisms in pathogenic fungi: from bench to bedside. Clin. Microbiol. Infect. 20 (Suppl. 6), 54–59 (2014).
Smith, K. D. et al. Increased antifungal drug resistance in clinical isolates of Cryptococcus neoformans in Uganda. Antimicrob. Agents Chemother. 59, 7197–7204 (2015).
Denning, D. W. & Bromley, M. J. Infectious disease. How to bolster the antifungal pipeline. Science 347, 1414–1416 (2015). This insightful discussion describes the potential needs and barriers to antifungal development.
Ostrosky-Zeichner, L., Casadevall, A., Galgiani, J. N., Odds, F. C. & Rex, J. H. An insight into the antifungal pipeline: selected new molecules and beyond. Nat. Rev. Drug Discov. 9, 719–727 (2010). By comparing reference 15 and this Review, the progress that has been made in the antifungal pipeline in the past decade can be determined.
Osherov, N. & Kontoyiannis, D. P. The anti-Aspergillus drug pipeline: is the glass half full or empty? Med. Mycol. 55, 118–124 (2017).
Odds, F. C., Brown, A. J. & Gow, N. A. Antifungal agents: mechanisms of action. Trends Microbiol. 11, 272–279 (2003).
Allen, D., Wilson, D., Drew, R. & Perfect, J. Azole antifungals: 35 years of invasive fungal infection management. Expert Rev. Anti Infect. Ther. 13, 787–798 (2015).
Hamill, R. J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 73, 919–934 (2013).
Holt, S. L. & Drew, R. H. Echinocandins: addressing outstanding questions surrounding treatment of invasive fungal infections. Am. J. Health Syst. Pharm. 68, 1207–1220 (2011).
Peyton, L. R., Gallagher, S. & Hashemzadeh, M. Triazole antifungals: a review. Drugs Today (Barc.) 51, 705–718 (2015).
Nett, J. E. & Andes, D. R. Antifungal agents: spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. North Am. 30, 51–83 (2016).
Andes, D. R. et al. Impact of treatment strategy on outcomes in patients with candidemia and other forms of invasive candidiasis: a patient-level quantitative review of randomized trials. Clin. Infect. Dis. 54, 1110–1122 (2012).
Bratton, E. W. et al. Comparison and temporal trends of three groups with cryptococcosis: HIV-infected, solid organ transplant, and HIV-negative/non-transplant. PLoS ONE 7, e43582 (2012).
Nyazika, T. K. et al. Cryptococcus neoformans population diversity and clinical outcomes of HIV-associated cryptococcal meningitis patients in Zimbabwe. J. Med. Microbiol. 65, 1281–1288 (2016).
Marr, K. A. et al. Combination antifungal therapy for invasive aspergillosis: a randomized trial. Ann. Intern. Med. 162, 81–89 (2015).
Perfect, J. R. Fungal virulence genes as targets for antifungal chemotherapy. Antimicrob. Agents Chemother. 40, 1577–1583 (1996).
Kitamura, A., Someya, K., Hata, M., Nakajima, R. & Takemura, M. Discovery of a small-molecule inhibitor of β-1,6-glucan synthesis. Antimicrob. Agents Chemother. 53, 670–677 (2009).
Baxter, B. K., DiDone, L., Ogu, D., Schor, S. & Krysan, D. J. Identification, in vitro activity and mode of action of phosphoinositide-dependent-1 kinase inhibitors as antifungal molecules. ACS Chem. Biol. 6, 502–510 (2011).
Breger, J. et al. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 3, e18 (2007).
Spitzer, M. et al. Cross-species discovery of syncretic drug combinations that potentiate the antifungal fluconazole. Mol. Syst. Biol. 7, 499 (2011).
Roemer, T. et al. Confronting the challenges of natural product-based antifungal discovery. Chem. Biol. 18, 148–164 (2011).
Tebbets, B. et al. Identification and characterization of antifungal compounds using a Saccharomyces cerevisiae reporter bioassay. PLoS ONE 7, e36021 (2012).
Krysan, D. J. & Didone, L. A high-throughput screening assay for small molecules that disrupt yeast cell integrity. J. Biomol. Screen. 13, 657–664 (2008).
Lamoth, F., Juvvadi, P. R. & Steinbach, W. J. Editorial: advances in Aspergillus fumigatus pathobiology. Front. Microbiol. 7, 43 (2016).
Juvvadi, P. R., Lee, S. C., Heitman, J. & Steinbach, W. J. Calcineurin in fungal virulence and drug resistance: prospects for harnessing targeted inhibition of calcineurin for an antifungal therapeutic approach. Virulence 8, 186–197 (2017).
Blankenship, J. R., Steinbach, W. J., Perfect, J. R. & Heitman, J. Teaching old drugs new tricks: reincarnation immunosuppressants as antifungal drugs. Curr. Opin. Investig. Drugs 4, 192–199 (2003).
Cowen, L. E. et al. Harnessing Hsp90 function as a powerful, broadly effective therapeutic strategy for fungal infectious disease. Proc. Natl Acad. Sci. USA 106, 2818–2823 (2009).
Pachl, J. et al. A randomized, blinded, multicenter trial of lipid-associated Amphotericin B alone versus in combination with an antibody-based inhibitor of heat shock protein 90 in patients with invasive candidiasis. Clin. Infect. Dis. 42, 1404–1413 (2006).
Nambu, M. et al. A calcineurin antifungal strategy with analogs of FK506. Bioorg. Med. Chem. Lett. http://dx.doi.org/10.1016/j.bmcl.2017.04.004 (2011).
Hast, M. A. et al. Structures of Cryptococcus neoformans protein farnesyltransferase reveal strategies for developing inhibitors that target fungal pathogens. J. Biol. Chem. 286, 35149–35162 (2011).
Mor, V. et al. Identification of a new class of antifungals targeting the synthesis of fungal sphingolipids. mBio 6, e00647 (2015).
Rollin-Pinheiro, R., Singh, A., Barreto-Bergter, E. & Del Poeta, M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 8, 1469–1484 (2016).
Perfect, J. R., Tenor, J. L., Miao, Y. & Brennan, R. G. Trehalose pathway as an antifungal target. Virulence 8, 143–149 (2016).
Petzold, E. W. et al. Characterization and regulation of the trehalose synthesis pathway and its importance in the pathogenicity of Cryptococcus neoformans. Infect. Immun. 74, 5877–5887 (2006).
Ngamskulrungroj, P. et al. The trehalose pathway: an integral part of virulence composite for Cryptococcus gattii. Infect. Immun. 77, 4584–4596 (2009).
Miao, Y. et al. Structures of trehalose-6-phosphate phosphatase from pathogenic fungi reveal the mechanisms of substrate recognition and catalysis. Proc. Natl Acad. Sci. USA 113, 7148–7153 (2016).
Cheah, H. L., Lim, V. & Sandai, D. Inhibitors of the glyoxylate cycle enzyme ICL1 in Candida albicans for potential use as antifungal agents. PLoS ONE 9, e95951 (2014).
Knauth, P. & Reichenbach, H. On the mechanism of action of the myxobacterial fungicide ambruticin. J. Antibiot. (Tokyo) 53, 1182–1190 (2000).
Levine, H. B., Ringel, S. M. & Cobb, J. M. Therapeutic properties of oral ambruticin (W7783) in experimental pulmonary coccidioidomycosis of mice. Chest 73, 202–206 (1978).
Shubitz, L. F. et al. Efficacy of ambruticin analogs in a murine model of coccidioidomycosis. Antimicrob. Agents Chemother. 50, 3467–3469 (2006).
Hagihara, K. et al. Fingolimod (FTY720) stimulates Ca2+/calcineurin signaling in fission yeast. PLoS ONE 8, e81907 (2013).
Gallo-Ebert, C. et al. Novel antifungal drug discovery based on targeting pathways regulating the fungus-conserved Upc2 transcription factor. Antimicrob. Agents Chemother. 58, 258–266 (2014).
Hein, K. Z. et al. Disulphide-reduced psoriasin is a human apoptosis-inducing broad-spectrum fungicide. Proc. Natl Acad. Sci. USA 112, 13039–13044 (2015).
Valiante, V. et al. Hitting the caspofungin salvage pathway of human-pathogenic fungi with the novel lasso peptide humidimycin (MDN-0010). Antimicrob. Agents Chemother. 59, 5145–5153 (2015).
Liu, N., Wang, C., Su, H., Zhang, W. & Sheng, C. Strategies in the discovery of novel antifungal scaffolds. Future Med. Chem. 8, 1435–1454 (2016).
Scorzoni, L. et al. Searching new antifungals: the use of in vitro and in vivo methods for evaluation of natural compounds. J. Microbiol. Methods 123, 68–78 (2016).
Nobrega, R. O., Teixeira, A. P., Oliveira, W. A., Lima, E. O. & Lima, I. O. Investigation of the antifungal activity of carvacrol against strains of Cryptococcus neoformans. Pharm. Biol. 54, 2591–2596 (2016).
Zuo, R. et al. In vitro antifungal and antibiofilm activities of halogenated quinoline analogues against Candida albicans and Cryptococcus neoformans. Int. J. Antimicrob. Agents 48, 208–211 (2016).
Ghannoum, M. A., Kim, H. G. & Long, L. Efficacy of aminocandin in the treatment of immunocompetent mice with haematogenously disseminated fluconazole-resistant candidiasis. J. Antimicrob. Chemother. 59, 556–559 (2007).
Warn, P. A., Sharp, A., Morrissey, G. & Denning, D. W. Activity of aminocandin (IP960; HMR3270) compared with amphotericin B, itraconazole, caspofungin and micafungin in neutropenic murine models of disseminated infection caused by itraconazole-susceptible and -resistant strains of Aspergillus fumigatus. Int. J. Antimicrob. Agents 35, 146–151 (2010).
Hanadate, T. et al. FR290581, a novel sordarin derivative: synthesis and antifungal activity. Bioorg. Med. Chem. Lett. 19, 1465–1468 (2009).
Hasenoehrl, A. et al. In vitro activity and in vivo efficacy of icofungipen (PLD-118), a novel oral antifungal agent, against the pathogenic yeast Candida albicans. Antimicrob. Agents Chemother. 50, 3011–3018 (2006).
Sigurgeirsson, B., van Rossem, K., Malahias, S. & Raterink, K. A phase II, randomized, double-blind, placebo-controlled, parallel group, dose-ranging study to investigate the efficacy and safety of 4 dose regimens of oral albaconazole in patients with distal subungual onychomycosis. J. Am. Acad. Dermatol. 69, 416–425 (2013).
Miller, J. L. et al. In vitro and in vivo efficacies of the new triazole albaconazole against Cryptococcus neoformans. Antimicrob. Agents Chemother. 48, 384–387 (2004).
Nakamura, I. et al. ASP2397: a novel antifungal agent produced by Acremonium persicinum MF-347833. J. Antibiot. (Tokyo) 70, 45–51 (2017).
Nakamura, I. et al. Discovery of a new antifungal agent ASP2397 using a silkworm model of Aspergillus fumigatus infection. J. Antibiot. (Tokyo) 70, 41–44 (2017).
Nishikawa, H. et al. T-2307, a novel arylamidine, is transported into Candida albicans by a high-affinity spermine and spermidine carrier regulated by Agp2. J. Antimicrob. Chemother. 71, 1845–1855 (2016).
Shibata, T. et al. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob. Agents Chemother. 56, 5892–5897 (2012).
Mitsuyama, J. et al. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob. Agents Chemother. 52, 1318–1324 (2008).
Yamada, E., Nishikawa, H., Nomura, N. & Mitsuyama, J. T-2307 shows efficacy in a murine model of Candida glabrata infection despite in vitro trailing growth phenomena. Antimicrob. Agents Chemother. 54, 3630–3634 (2010).
Koselny, K. et al. The celecoxib derivative AR-12 has broad spectrum antifungal activity in vitro and improves the activity of fluconazole in a murine model of cryptococcosis. Antimicrob. Agents Chemother. 60, 7115–7127 (2016).
Koselny, K. et al. Antitumor/antifungal celecoxib derivative AR-12 is a non-nucleoside inhibitor of the ANL-family adenylating enzyme acetyl CoA synthetase. ACS Infect. Dis. 2, 268–280 (2016).
Richard, M. L. & Plaine, A. Comprehensive analysis of glycosylphosphatidylinositol-anchored proteins in Candida albicans. Eukaryot. Cell 6, 119–133 (2007).
Watanabe, N. A. et al. E1210, a new broad-spectrum antifungal, suppresses Candida albicans hyphal growth through inhibition of glycosylphosphatidylinositol biosynthesis. Antimicrob. Agents Chemother. 56, 960–971 (2012).
McLellan, C. A. et al. Inhibiting GPI anchor biosynthesis in fungi stresses the endoplasmic reticulum and enhances immunogenicity. ACS Chem. Biol. 7, 1520–1528 (2012).
Hata, K. et al. Efficacy of oral E1210, a new broad-spectrum antifungal with a novel mechanism of action, in murine models of candidiasis, aspergillosis, and fusariosis. Antimicrob. Agents Chemother. 55, 4543–4551 (2011).
Wiederhold, N. P. et al. The investigational agent E1210 is effective in treatment of experimental invasive candidiasis caused by resistant Candida albicans. Antimicrob. Agents Chemother. 59, 690–692 (2015).
Miyazaki, M. et al. In vitro activity of E1210, a novel antifungal, against clinically important yeasts and molds. Antimicrob. Agents Chemother. 55, 4652–4658 (2011).
Shubitz, L. F. et al. Modeling nikkomycin Z dosing and pharmacology in murine pulmonary coccidioidomycosis preparatory to phase 2 clinical trials. J. Infect. Dis. 209, 1949–1954 (2014).
Hector, R. F., Zimmer, B. L. & Pappagianis, D. Evaluation of nikkomycins X and Z in murine models of coccidioidomycosis, histoplasmosis, and blastomycosis. Antimicrob. Agents Chemother. 34, 587–593 (1990).
Fortwendel, J. R. et al. Differential effects of inhibiting chitin and 1,3-β-D-glucan synthesis in ras and calcineurin mutants of Aspergillus fumigatus. Antimicrob. Agents Chemother. 53, 476–482 (2009).
Lilla, E. A. & Yokoyama, K. Carbon extension in peptidylnucleoside biosynthesis by radical SAM enzymes. Nat. Chem. Biol. 12, 905–907 (2016).
Pfaller, M. A. et al. Activity of MGCD290, a Hos2 histone deacetylase inhibitor, in combination with azole antifungals against opportunistic fungal pathogens. J. Clin. Microbiol. 47, 3797–3804 (2009).
Pfaller, M. A., Rhomberg, P. R., Messer, S. A. & Castanheira, M. In vitro activity of a Hos2 deacetylase inhibitor, MGCD290, in combination with echinocandins against echinocandin-resistant Candida species. Diagn. Microbiol. Infect. Dis. 81, 259–263 (2015).
Cowen, L. E. The fungal Achilles' heel: targeting Hsp90 to cripple fungal pathogens. Curr. Opin. Microbiol. 16, 377–384 (2013).
Lamoth, F., Alexander, B. D., Juvvadi, P. R. & Steinbach, W. J. Antifungal activity of compounds targeting the Hsp90-calcineurin pathway against various mould species. J. Antimicrob. Chemother. 70, 1408–1411 (2015).
Bugli, F. et al. Human monoclonal antibody-based therapy in the treatment of invasive candidiasis. Clin. Dev. Immunol. 2013, 403121 (2013).
Louie, A. et al. Dose range evaluation of Mycograb C28Y variant, a human recombinant antibody fragment to heat shock protein 90, in combination with amphotericin B-desoxycholate for treatment of murine systemic candidiasis. Antimicrob. Agents Chemother. 55, 3295–3304 (2011).
Aeed, P. A. Young, C. L., Nagiec, M. M. & Elhammer, A. P. Inhibition of inositol phosphorylceramide synthase by the cyclic peptide aureobasidin A. Antimicrob. Agents Chemother. 53, 496–504 (2009).
Kurome, T., Inoue, T., Takesako, K. & Kato, I. Syntheses of antifungal aureobasidin A analogs with alkyl chains for structure-activity relationship. J. Antibiot. 51, 359–367 (1998).
Kumaresan, P. R. et al. Bioengineering T cells to target carbohydrate to treat opportunistic fungal infection. Proc. Natl Acad. Sci. USA 111, 10660–10665 (2014).
Arikan, S. & Rex, J. H. Nystatin LF (Aronex/Abbott). Curr. Opin. Investig. Drugs 2, 488–495 (2001).
Janout, V., Bienvenu, C., Schell, W., Perfect, J. R. & Regen, S. L. Molecular umbrella-amphotericin B conjugates. Bioconjug. Chem. 25, 1408–1411 (2014).
Yang, Z. et al. Development and characterization of amphotericin B nanosuspensions for oral administration through a simple top-down method. Curr. Pharm. Biotechnol. 15, 569–576 (2014).
Halperin, A. et al. Novel water-soluble amphotericin B-PEG conjugates with low toxicity and potent in vivo efficacy. J. Med. Chem. 59, 1197–1206 (2016).
Ickowicz, D. E. et al. Activity, reduced toxicity, and scale-up synthesis of amphotericin B-conjugated polysaccharide. Biomacromolecules 15, 2079–2089 (2014).
Jung, S. H. et al. Amphotericin B-entrapping lipid nanoparticles and their in vitro and in vivo characteristics. Eur. J. Pharm. Sci. 37, 313–320 (2009).
Janout, V. et al. Taming amphotericin B. Bioconjug. Chem. 26, 2021–2024 (2015).
Delmas, G. et al. Efficacy of orally delivered cochleates containing amphotericin B in a murine model of aspergillosis. Antimicrob. Agents Chemother. 46, 2704–2707 (2002).
Ong, V. et al. Preclinical evaluation of the stability, safety and efficacy of CD101, a novel echinocandin. Antimicrob. Agents Chemother. 60, 6872–6879 (2016).
Pfaller, M. A., Messer, S. A., Rhomberg, P. R., Jones, R. N. & Castanheira, M. Activity of a long-acting echinocandin, CD101, determined using CLSI and EUCAST reference methods, against Candida and Aspergillus spp., including echinocandin- and azole-resistant isolates. J. Antimicrob. Chemother. 71, 2868–2873 (2016).
Walker, S. S. et al. Discovery of a novel class of orally active antifungal β-1,3-d-glucan synthase inhibitors. Antimicrob. Agents Chemother. 55, 5099–5106 (2011).
Gebremariam, T. et al. VT-1161 protects immunosuppressed mice from Rhizopus arrhizus var. arrhizus infection. Antimicrob. Agents Chemother. 59, 7815–7817 (2015).
Warrilow, A. G. et al. The clinical candidate VT-1161 is a highly potent inhibitor of Candida albicans CYP51 but fails to bind the human enzyme. Antimicrob. Agents Chemother. 58, 7121–7127 (2014).
Gupta, A. K. & Studholme, C. Novel investigational therapies for onychomycosis: an update. Expert Opin. Investig. Drugs 25, 297–305 (2016).
Lockhart, S. R. et al. The investigational fungal Cyp51 inhibitor VT-1129 demonstrates potent in vitro activity against Cryptococcus neoformans and Cryptococcus gattii. Antimicrob. Agents Chemother. 60, 2528–2531 (2016).
Butts, A. & Krysan, D. J. Antifungal drug discovery: something old and something new. PLoS Pathog. 8, e1002870 (2012).
Christenson, J. C., Shalit, I., Welch, D. F., Guruswamy, A. & Marks, M. I. Synergistic action of amphotericin B and rifampin against Rhizopus species. Antimicrob. Agents Chemother. 31, 1775–1778 (1987).
Liu, S. et al. Synergistic effect of fluconazole and calcium channel blockers against resistant Candida albicans. PLoS ONE 11, e0150859 (2016).
Coelho, C. & Casadevall, A. Cryptococcal therapies and drug targets: the old, the new and the promising. Cell. Microbiol. 18, 792–799 (2016).
Shirazi, F. & Kontoyiannis, D. P. The calcineurin pathway inhibitor tacrolimus enhances the in vitro activity of azoles against Mucorales via apoptosis. Eukaryot. Cell 12, 1225–1234 (2013).
Husain, S., Wagner, M. & Singh, N. Cryptococcus neoformans infection in organ transplant recipients: variables influencing clinical characteristics and outcome. Emerg. Infect. Dis. 7, 375–381 (2001).
Duke, S. S. & Fromtling, R. A. Effects of diethyl-stilbestrol and cyclophosphamide on the pathogenesis of experimental Cryptococcus neoformans infections. Sabouraudia 22, 125–135 (1984).
Mohr, J. A., Tatem, B. A., Long, H., Muchmore, H. G. & Felton, F. G. Increased susceptibility of Cryptococcus neoformans to amphotericin B in the presence of steroids. Sabouraudia 11, 140–142 (1973).
Butts, A. et al. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 5, e00765-13 (2014).
Dolan, K. et al. Antifungal activity of tamoxifen: in vitro and in vivo activities and mechanistic characterization. Antimicrob. Agents Chemother. 53, 3337–3346 (2009).
Zhai, B., Wu, C., Wang, L., Sachs, M. S. & Lin, X. The antidepressant sertraline provides a promising therapeutic option for neurotropic cryptococcal infections. Antimicrob. Agents Chemother. 56, 3758–3766 (2012).
Rhein, J. et al. Efficacy of adjunctive sertraline for the treatment of HIV-associated cryptococcal meningitis: an open-label dose-ranging study. Lancet Infect. Dis. 16, 809–818 (2016).
Del Poeta, M. et al. Comparison of in vitro activities of camptothecin and nitidine derivatives against fungal and cancer cells. Antimicrob. Agents Chemother. 43, 2862–2868 (1999).
Cardenas, M. E. et al. Antifungal activities of antineoplastic agents: Saccharomyces cerevisiae as a model system to study drug action. Clin. Microbiol. Rev. 12, 583–611 (1999).
Widmer, F. et al. Hexadecylphosphocholine (miltefosine) has broad-spectrum fungicidal activity and is efficacious in a mouse model of cryptococcosis. Antimicrob. Agents Chemother. 50, 414–421 (2006).
Wiederhold, N. P. et al. Limited activity of miltefosine in murine models of cryptococcal meningoencephalitis and disseminated cryptococcosis. Antimicrob. Agents Chemother. 57, 745–750 (2013).
Pace, J. R. et al. Repurposing the clinically efficacious antifungal agent itraconazole as an anticancer chemotherapeutic. J. Med. Chem. 59, 3635–3649 (2016).
Abuhelwa, A. Y., Foster, D. J., Mudge, S., Hayes, D. & Upton, R. N. Population pharmacokinetic modeling of itraconazole and hydroxyitraconazole for oral SUBA-itraconazole and sporanox capsule formulations in healthy subjects in fed and fasted states. Antimicrob. Agents Chemother. 59, 5681–5696 (2015).
Le, J. & Schiller, D. S. Aerosolized delivery of antifungal agents. Curr. Fungal Infect. Rep. 4, 96–102 (2010).
Pornputtapitak, W., El-Gendy, N. & Berkland, C. NanoCluster itraconazole formulations provide a potential engineered drug particle approach to generate effective dry powder aerosols. J. Aerosol Med. Pulm. Drug Deliv. 28, 341–352 (2015).
Walraven, C. J. & Lee, S. A. Antifungal lock therapy. Antimicrob. Agents Chemother. 57, 1–8 (2013).
Tramsen, L. et al. Clinical-scale generation of multi-specific anti-fungal T cells targeting Candida, Aspergillus and mucormycetes. Cytotherapy 15, 344–351 (2013).
Zhao, X. et al. JNK1 negatively controls antifungal innate immunity by suppressing CD23 expression. Nat. Med. 23, 337–346 (2017).
Galgiani, J. N. Vaccines to prevent systemic mycoses: holy grails meet translational realities. J. Infect. Dis. 197, 938–940 (2008). This article discusses the potential for antifungal vaccines.
Casadevall, A. & Pirofski, L. A. Feasibility and prospects for a vaccine to prevent cryptococcosis. Med. Mycol. 43, 667–680 (2005).
Cutler, J. E., Deepe, G. S. Jr & Klein, B. S. Advances in combating fungal diseases: vaccines on the threshold. Nat. Rev. Microbiol. 5, 13–28 (2007).
Levitz, S. M. Aspergillus vaccines: hardly worth studying or worthy of hard study? Med. Mycol. 55, 103–108 (2017).
Upadhya, R. et al. Induction of protective immunity to cryptococcal infection in mice by a heat-killed, chitosan-deficient strain of Cryptococcus neoformans. mBio 7, e00547-16 (2016).
Mor, V. et al. Glucosylceramide administration as a vaccination strategy in mouse models of cryptococcosis. PLoS ONE 11, e0153853 (2016).
Wormley, F. L. Jr, Perfect, J. R., Steele, C. & Cox, G. M. Protection against cryptococcosis by using a murine gamma interferon-producing Cryptococcus neoformans strain. Infect. Immun. 75, 1453–1462 (2007).
Pappagianis, D. Evaluation of the protective efficacy of the killed Coccidioides immitis spherule vaccine in humans. The Valley Fever Vaccine Study Group. Am. Rev. Respir. Dis. 148, 656–660 (1993).
Edwards, J. E. Jr. Fungal cell wall vaccines: an update. J. Med. Microbiol. 61, 895–903 (2012).
Larsen, R. A. et al. Phase I evaluation of the safety and pharmacokinetics of murine-derived anticryptococcal antibody 18B7 in subjects with treated cryptococcal meningitis. Antimicrob. Agents Chemother. 49, 952–958 (2005).
Bryan, R. A. et al. Toward developing a universal treatment for fungal disease using radioimmunotherapy targeting common fungal antigens. Mycopathologia 173, 463–471 (2012).
Jarvis, J. et al. Adjunctive interferon-γ immnotherapy for the treatment of HIV-associated cryptococcal meningitis: a randomized controlled trial. AIDS 26, 1105–1113 (2012).
Pappas, P. G. et al. Recombinant interferon-γ 1b as adjunctive therapy for AIDS-related acute cryptococcal meningitis. J. Infect. Dis. 189, 2185–2191 (2004).
Rodero, L. et al. In vitro susceptibility studies of Cryptococcus neoformans isolated from patients with no clinical response to amphotericin B therapy. J. Antimicrob. Chemother. 45, 239–242 (2000).
Sanglard, D. & Odds, F. C. Resistance of Candida species to antifungal agents: molecular mechanisms and clinical consequences. Lancet Infect. Dis. 2, 73–85 (2002).
Verweij, P. E., Chowdhary, A., Melchers, W. J. & Meis, J. F. Azole resistance in Aspergillus fumigatus: can we retain the clinical use of mold-active antifungal azoles? Clin. Infect. Dis. 62, 362–368 (2016).
Alexander, B. D. et al. Increasing echinocandin resistance in Candida glabrata: clinical failure correlates with presence of FKS mutations and elevated minimum inhibitory concentrations. Clin. Infect. Dis. 56, 1724–1732 (2013).
Healey, K. R. et al. Prevalent mutator genotype identified in fungal pathogen Candida glabrata promotes multi-drug resistance. Nat. Commun. 7, 11128 (2016).
Edlind, T. D. & Katiyar, S. K. Mutational analysis of flucytosine resistance in Candida glabrata. Antimicrob. Agents Chemother. 54, 4733–4738 (2010).
Acknowledgements
The author has research support from Public Health Service Grants (AI73896, AI04533, AI93257).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The author declares research grants, consulting arrangements, honorariums and advisory committees from the following companies: Merck & Co., Astellas, Pfizer Inc, F2G, Vical, Arno Therapeutics, Cidara, Scynexis, Viamet, Matinas BioPharma, Amplyx Pharmaceuticals and Teva Pharmaceutical Industries.
Related links
DATABASES
FURTHER INFORMATION
Glossary
- Mycetomas
-
Chronic fungal infections of skin and soft tissue.
- T2 MRI
-
(Magnetic resonance imaging). A testing system from T2 Biosystems that uses advances in nanotechnology and molecular biology to detect microorganisms directly from body fluids.
- GAIN Act
-
The Generating Antibiotic Incentives Now (GAIN) Act by the US Federal Government is legislation that aims to stimulate antimicrobial discovery.
- Orphan Drug Act
-
The Orphan Drug Act enables the US Food and Drug Administration (FDA) to designate a treatment as being developed for a rare disease upon request from a sponsor.
- Fast Track
-
Fast Track is a US Food and Drug Administration (FDA) designation for expedited review of drugs to fill unmet medical needs, as requested by a drug company.
- Nosocomial
-
Hospital-based.
- Virulence composite
-
The genetic and phenotypic traits that encompass pathogenicity.
Rights and permissions
About this article
Cite this article
Perfect, J. The antifungal pipeline: a reality check. Nat Rev Drug Discov 16, 603–616 (2017). https://doi.org/10.1038/nrd.2017.46
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2017.46
This article is cited by
-
Targeting yeast topoisomerase II by imidazo and triazoloacridinone derivatives resulting in their antifungal activity
Scientific Reports (2024)
-
In vitro activity of the anthelmintic drug niclosamide against Sporothrix spp. strains with distinct genetic and antifungal susceptibility backgrounds
Brazilian Journal of Microbiology (2024)
-
Utilizing the above-ground extract of Paris polyphylla as a natural antioxidant and antimicrobial additive in soap formulation
Biomass Conversion and Biorefinery (2024)
-
A promising antifungal lipopeptide from Bacillus subtilis: its characterization and insight into the mode of action
Applied Microbiology and Biotechnology (2024)
-
Dehydrocostus lactone inhibits Candida albicans growth and biofilm formation
AMB Express (2023)