Key Points
-
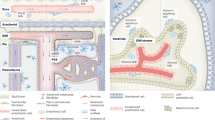
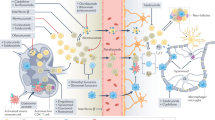
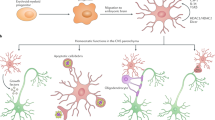
Brain myeloid cells are a diverse group of mononuclear cells that mediate the local immune response during development, health and brain diseases. They consist of endogenous microglia, CNS-resident macrophages and monocytes that infiltrate the diseased CNS, each of them having specific disease-related functions.
-
Mutations in microglial-related genes can have a significant impact on the functions of these cells and are linked to various disorders. Primary microgliopathies are usually caused by a single gene mutation, whereby the lack of a distinct microglial-associated gene results in microglia dysfunction accompanied by neural damage.
-
In secondary myeloid cell brain disorders, myeloid cells undergo a shift towards a disease-specific phenotype that potentially contributes to the chronicity of these diseases.
-
There are various lines of evidence showing significant differences in myeloid cell function between mice and humans. From a drug discovery perspective, it is crucial to understand whether findings in mice also apply to brain myeloid cells in humans.
-
Brain myeloid cells are increasingly being recognized as promising potential targets for the treatment of CNS disorders. Several pharmaceutical drug development programmes targeting brain myeloid cells have been initiated, with more anticipated in the near future.
Abstract
Myeloid cells of the central nervous system (CNS), which include parenchymal microglia, macrophages at CNS interfaces and monocytes recruited from the circulation during disease, are increasingly being recognized as targets for therapeutic intervention in neurological and psychiatric diseases. The origin of these cells in the immune system distinguishes them from ectodermal neurons and other glia and endows them with potential drug targets distinct from classical CNS target groups. However, despite the identification of several promising therapeutic approaches and molecular targets, no agents directly targeting these cells are currently available. Here, we assess strategies for targeting CNS myeloid cells and address key issues associated with their translation into the clinic.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Prinz, M. & Priller, J. Microglia and brain macrophages in the molecular age: from origin to neuropsychiatric disease. Nat. Rev. Neurosci. 15, 300–312 (2014).
Ransohoff, R. M. & Cardona, A. E. The myeloid cells of the central nervous system parenchyma. Nature 468, 253–262 (2010).
Kettenmann, H., Hanisch, U.-K., Noda, M. & Verkhratsky, A. Physiology of microglia. Physiol. Rev. 91, 461–553 (2011).
Gautier, E. L. et al. Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat. Immunol. 13, 1118–1128 (2012). A landmark paper providing convincing evidence of the diversity of the myeloid cell system, including some brain myeloid cells.
Gosselin, D. et al. Environment drives selection and function of enhancers controlling tissue-specific macrophage identities. Cell 159, 1327–1340 (2014).
Lavin, Y. et al. Tissue-resident macrophage enhancer landscapes are shaped by the local microenvironment. Cell 159, 1312–1326 (2014). References 5 and 6 both show independently that myeloid cell diversity is shaped by the environment, which is reflected by changes in the epigenome.
Butovsky, O. et al. Identification of a unique TGF-β-dependent molecular and functional signature in microglia. Nat. Neurosci. 17, 131–143 (2014). The paper identifies various microglia-specific genes.
Erny, D. et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 18, 965–977 (2015). A landmark paper showing that the gut microbiota is important for proper microglia function.
Hickman, S. E. et al. The microglial sensome revealed by direct RNA sequencing. Nat. Neurosci. 16, 1896–1905 (2013). The first thorough analysis of the receptor profile of mature microglia from the adult brain.
Davalos, D. et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 8, 752–758 (2005).
Nimmerjahn, A., Kirchhoff, F. & Helmchen, F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science 308, 1314–1318 (2005). One of the most influential papers on microglia of the past decade, this study provides initial proof that microglial processes constantly move and survey their environment.
Tremblay, M.-È., Lowery, R. L. & Majewska, A. K. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 8, e1000527 (2010).
Wake, H., Moorhouse, A. J., Jinno, S., Kohsaka, S. & Nabekura, J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J. Neurosci. 29, 3974–3980 (2009). References 12 and 13 are landmark papers showing convincingly that microglia have brief contacts with active synapses, indicating their role in modulating synaptic activity.
Roumier, A. et al. Impaired synaptic function in the microglial KARAP/DAP12-deficient mouse. J. Neurosci. 24, 11421–11428 (2004).
Zhan, Y. et al. Deficient neuron–microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 17, 400–406 (2014).
Marín-Teva, J. L. et al. Microglia promote the death of developing Purkinje cells. Neuron 41, 535–547 (2004).
Ueno, M. et al. Layer V cortical neurons require microglial support for survival during postnatal development. Nat. Neurosci. 16, 543–551 (2013).
Dijkstra, C. D., Döpp, E. A., Joling, P. & Kraal, G. The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in the rat recognized by monoclonal antibodies ED1, ED2 and ED3. Immunology 54, 589–599 (1985).
Serrats, J. et al. Dual roles for perivascular macrophages in immune-to-brain signaling. Neuron 65, 94–106 (2010). One of the few papers that thoroughly addresses the function of perivascular macrophages.
Auffray, C. et al. CX3CR1+ CD115+ CD135+ common macrophage/DC precursors and the role of CX3CR1 in their response to inflammation. J. Exp. Med. 206, 595–606 (2009).
Geissmann, F., Gordon, S., Hume, D. A., Mowat, A. M. & Randolph, G. J. Unravelling mononuclear phagocyte heterogeneity. Nat. Rev. Immunol. 10, 453–460 (2010).
Mizutani, M. et al. The fractalkine receptor but not CCR2 is present on microglia from embryonic development throughout adulthood. J. Immunol. 188, 29–36 (2012).
Saederup, N. et al. Selective chemokine receptor usage by central nervous system myeloid cells in CCR2-red fluorescent protein knock-in mice. PLoS ONE 5, e13693 (2010).
Ginhoux, F. et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 330, 841–845 (2010).
Kierdorf, K. et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 16, 273–280 (2013). A complete description of the microglia progenitor and important transcription factors in microglia differentiation.
Schulz, C. et al. A lineage of myeloid cells independent of Myb and hematopoietic stem cells. Science 336, 86–90 (2012).
Ajami, B., Bennett, J. L., Krieger, C., Tetzlaff, W. & Rossi, F. M. V. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 10, 1538–1543 (2007). A landmark paper showing that there is no regular exchange of the microglia population from outside the brain.
Mildner, A. et al. Microglia in the adult brain arise from Ly-6ChiCCR2+ monocytes only under defined host conditions. Nat. Neurosci. 10, 1544–1553 (2007). This study describes how local conditioning is required for bone marrow cell engraftment into the CNS.
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Goldmann, T. et al. A new type of microglia gene targeting shows TAK1 to be pivotal in CNS autoimmune inflammation. Nat. Neurosci. 16, 1618–1626 (2013).
Yona, S. et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38, 79–91 (2013). References 30 and 31 are the first description of microglia-specific Cre lines.
Lambertsen, K. L., Biber, K. & Finsen, B. Inflammatory cytokines in experimental and human stroke. J. Cereb. Blood Flow Metab. 32, 1677–1698 (2012).
Prinz, M., Priller, J., Sisodia, S. S. & Ransohoff, R. M. Heterogeneity of CNS myeloid cells and their roles in neurodegeneration. Nat. Neurosci. 14, 1227–1235 (2011).
Kreutzberg, G. W. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 19, 312–318 (1996).
Streit, W. J., Walter, S. A. & Pennell, N. A. Reactive microgliosis. Prog. Neurobiol. 57, 563–581 (1999).
Tremblay, M.-È. et al. The role of microglia in the healthy brain. J. Neurosci. 31, 16064–16069 (2011).
Sierra, A., Tremblay, M.-È. & Wake, H. Never-resting microglia: physiological roles in the healthy brain and pathological implications. Front. Cell. Neurosci. 8, 240 (2014).
Rademakers, R. et al. Mutations in the colony stimulating factor 1 receptor (CSF1R) gene cause hereditary diffuse leukoencephalopathy with spheroids. Nat. Genet. 44, 200–205 (2012).
Goldmann, T. et al. USP18 lack in microglia causes destructive interferonopathy of the mouse brain. EMBO J. 34, 1612–1629 (2015).
Chen, S.-K. et al. Hematopoietic origin of pathological grooming in Hoxb8 mutant mice. Cell 141, 775–785 (2010).
Takahashi, K., Prinz, M., Stagi, M., Chechneva, O. & Neumann, H. TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis. PLoS Med. 4, e124 (2007).
Bradshaw, E. M. et al. CD33 Alzheimer's disease locus: altered monocyte function and amyloid biology. Nat. Neurosci. 16, 848–850 (2013).
Griciuc, A. et al. Alzheimer's disease risk gene CD33 inhibits microglial uptake of amyloid β. Neuron 78, 631–643 (2013).
Guerreiro, R. J. et al. Using exome sequencing to reveal mutations in TREM2 presenting as a frontotemporal dementia-like syndrome without bone involvement. JAMA Neurol. 70, 78–84 (2013).
De Jager, P. L. et al. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat. Genet. 41, 776–782 (2009).
International Multiple Sclerosis Genetics Consortium. The genetic association of variants in CD6, TNFRSF1A and IRF8 to multiple sclerosis: a multicenter case–control study. PLoS ONE 6, e18813 (2011).
Djukic, M. et al. Circulating monocytes engraft in the brain, differentiate into microglia and contribute to the pathology following meningitis in mice. Brain J. Neurol. 129, 2394–2403 (2006).
Malm, T. M. et al. Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to β-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol. Dis. 18, 134–142 (2005).
Priller, J. et al. Early and rapid engraftment of bone marrow-derived microglia in scrapie. J. Neurosci. 26, 11753–11762 (2006).
Solomon, J. N. et al. Origin and distribution of bone marrow-derived cells in the central nervous system in a mouse model of amyotrophic lateral sclerosis. Glia 53, 744–753 (2006).
Mildner, A. et al. Distinct and non-redundant roles of microglia and myeloid subsets in mouse models of Alzheimer's disease. J. Neurosci. 31, 11159–11171 (2011).
Biffi, A. et al. Lentiviral hematopoietic stem cell gene therapy benefits metachromatic leukodystrophy. Science 341, 1233158 (2013).
Cartier, N. et al. Hematopoietic stem cell gene therapy with a lentiviral vector in X-linked adrenoleukodystrophy. Science 326, 818–823 (2009).
Kierdorf, K., Katzmarski, N., Haas, C. A. & Prinz, M. Bone marrow cell recruitment to the brain in the absence of irradiation or parabiosis bias. PLoS ONE 8, e58544 (2013).
Biber, K., Owens, T. & Boddeke, E. What is microglia neurotoxicity (not)? Glia 62, 841–854 (2014).
Hellwig, S., Heinrich, A. & Biber, K. The brain's best friend: microglial neurotoxicity revisited. Front. Cell. Neurosci. 7, 71 (2013).
Sierra, A. et al. Surveillance, phagocytosis, and inflammation: how never-resting microglia influence adult hippocampal neurogenesis. Neural Plast. 2014, 610343 (2014).
Sierra, A., Abiega, O., Shahraz, A. & Neumann, H. Janus-faced microglia: beneficial and detrimental consequences of microglial phagocytosis. Front. Cell. Neurosci. 7, 6 (2013).
Block, M. L., Zecca, L. & Hong, J.-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat. Rev. Neurosci. 8, 57–69 (2007).
Benveniste, E. N. Role of macrophages/microglia in multiple sclerosis and experimental allergic encephalomyelitis. J. Mol. Med. Berl. Ger. 75, 165–173 (1997).
Goldmann, T. & Prinz, M. Role of microglia in CNS autoimmunity. Clin. Dev. Immunol. 2013, 208093 (2013).
Ellrichmann, G. et al. Constitutive activity of NF-κB in myeloid cells drives pathogenicity of monocytes and macrophages during autoimmune neuroinflammation. J. Neuroinflamm. 9, 15 (2012).
Vainchtein, I. D. et al. In acute experimental autoimmune encephalomyelitis, infiltrating macrophages are immune activated, whereas microglia remain immune suppressed. Glia 62, 1724–1735 (2014).
Yamasaki, R. et al. Differential roles of microglia and monocytes in the inflamed central nervous system. J. Exp. Med. 211, 1533–1549 (2014). References 63 and 64 provide evidence for the different roles of microglia versus peripheral monocytes in the EAE brain.
Evans, T. A. et al. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp. Neurol. 254, 109–120 (2014).
Olah, M. et al. Identification of a microglia phenotype supportive of remyelination. Glia 60, 306–321 (2012).
Lambertsen, K. L. et al. Microglia protect neurons against ischemia by synthesis of tumor necrosis factor. J. Neurosci. 29, 1319–1330 (2009).
Chen, Z. & Trapp, B. D. Microglia and neuroprotection. J. Neurochem. http://dx.doi.org/10.1111/jnc.13062 (2015).
Mosher, K. I. & Wyss-Coray, T. Microglial dysfunction in brain aging and Alzheimer's disease. Biochem. Pharmacol. 88, 594–604 (2014).
Coull, J. A. M. et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005).
Inoue, K. & Tsuda, M. P2X4 receptors of microglia in neuropathic pain. CNS Neurol. Disord. Drug Targets 11, 699–704 (2012).
Butovsky, O. et al. Targeting miR-155 restores abnormal microglia and attenuates disease in SOD1 mice. Ann. Neurol. 77, 75–99 (2015).
Moore, C. S. et al. P2Y12 expression and function in alternatively activated human microglia. Neurol. Neuroimmunol. Neuroinflamm. 2, e80 (2015).
Verheijden, S. et al. Identification of a chronic non-neurodegenerative microglia activation state in a mouse model of peroxisomal β-oxidation deficiency. Glia 63, 1606–1620 (2015).
Getts, D. R. Editorial to special issue: monocytes in homeostasis and disease. Cell. Immunol. 291, 1–2 (2014).
Serbina, N. V. & Pamer, E. G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat. Immunol. 7, 311–317 (2006).
Ajami, B., Bennett, J. L., Krieger, C., McNagny, K. M. & Rossi, F. M. V. Infiltrating monocytes trigger EAE progression, but do not contribute to the resident microglia pool. Nat. Neurosci. 14, 1142–1149 (2011).
Getts, D. R. et al. Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci. Transl. Med. 6, 219ra7 (2014).
Schwartz, M. & Baruch, K. The resolution of neuroinflammation in neurodegeneration: leukocyte recruitment via the choroid plexus. EMBO J. 33, 7–22 (2014).
Hawkes, C. A. & McLaurin, J. Selective targeting of perivascular macrophages for clearance of β-amyloid in cerebral amyloid angiopathy. Proc. Natl Acad. Sci. USA 106, 1261–1266 (2009).
Hickey, W. F., Ueno, K., Hiserodt, J. C. & Schmidt, R. E. Exogenously-induced, natural killer cell-mediated neuronal killing: a novel pathogenetic mechanism. J. Exp. Med. 176, 811–817 (1992).
Unger, E. R. et al. Male donor-derived cells in the brains of female sex-mismatched bone marrow transplant recipients: a Y-chromosome specific in situ hybridization study. J. Neuropathol. Exp. Neurol. 52, 460–470 (1993).
Elmore, M. R. P. et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 82, 380–397 (2014). A landmark paper showing the potential of CSF1R antagonists to deplete microglia in vivo.
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Steinman, L. Multiple sclerosis: a coordinated immunological attack against myelin in the central nervous system. Cell 85, 299–302 (1996).
Lutz, S. E. et al. Contribution of pannexin1 to experimental autoimmune encephalomyelitis. PLoS ONE 8, e66657 (2013).
Matute, C. et al. P2X7 receptor blockade prevents ATP excitotoxicity in oligodendrocytes and ameliorates experimental autoimmune encephalomyelitis. J. Neurosci. 27, 9525–9533 (2007).
Chen, L. & Brosnan, C. F. Exacerbation of experimental autoimmune encephalomyelitis in P2X7R−/− mice: evidence for loss of apoptotic activity in lymphocytes. J. Immunol. 176, 3115–3126 (2006).
Sharp, A. J. et al. P2X7 deficiency suppresses development of experimental autoimmune encephalomyelitis. J. Neuroinflamm. 5, 33 (2008).
Mildner, A. et al. CCR2+Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain J. Neurol. 132, 2487–2500 (2009).
Moreno, M. et al. Conditional ablation of astroglial CCL2 suppresses CNS accumulation of M1 macrophages and preserves axons in mice with MOG peptide EAE. J. Neurosci. 34, 8175–8185 (2014).
Inagaki-Ohara, K., Kondo, T., Ito, M. & Yoshimura, A. SOCS, inflammation, and cancer. JAKSTAT 2, e24053 (2013).
Qin, H. et al. Signal transducer and activator of transcription-3/suppressor of cytokine signaling-3 (STAT3/SOCS3) axis in myeloid cells regulates neuroinflammation. Proc. Natl Acad. Sci. USA 109, 5004–5009 (2012).
Ponomarev, E. D., Veremeyko, T., Barteneva, N., Krichevsky, A. M. & Weiner, H. L. MicroRNA-124 promotes microglia quiescence and suppresses EAE by deactivating macrophages via the C/EBP-α–PU.1 pathway. Nat. Med. 17, 64–70 (2011). The first paper indicating that miRNAs are potential drug targets in brain myeloid cells.
Moore, C. S. et al. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann. Neurol. 74, 709–720 (2013).
Murugaiyan, G., Beynon, V., Mittal, A., Joller, N. & Weiner, H. L. Silencing microRNA-155 ameliorates experimental autoimmune encephalomyelitis. J. Immunol. 187, 2213–2221 (2011).
Mc Guire, C., Prinz, M., Beyaert, R. & van Loo, G. Nuclear factor kappa B (NF-κB) in multiple sclerosis pathology. Trends Mol. Med. 19, 604–613 (2013).
Malik, M. et al. CD33 Alzheimer's risk-altering polymorphism, CD33 expression, and exon 2 splicing. J. Neurosci. 33, 13320–13325 (2013).
Bard, F. et al. Peripherally administered antibodies against amyloidβ-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 6, 916–919 (2000).
Janus, C. et al. Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408, 979–982 (2000).
Adolfsson, O. et al. An effector-reduced anti-β-amyloid (Aβ) antibody with unique Aβ binding properties promotes neuroprotection and glial engulfment of Aβ. J. Neurosci. 32, 9677–9689 (2012).
Kleinberger, G. et al. TREM2 mutations implicated in neurodegeneration impair cell surface transport and phagocytosis. Sci. Transl. Med. 6, 243ra86 (2014).
Jiang, T. et al. Upregulation of TREM2 ameliorates neuropathology and rescues spatial cognitive impairment in a transgenic mouse model of Alzheimer's disease. Neuropsychopharmacology 39, 2949–2962 (2014).
Jay, T. R. et al. TREM2 deficiency eliminates TREM2+ inflammatory macrophages and ameliorates pathology in Alzheimer's disease mouse models. J. Exp. Med. 212, 287–295 (2015).
Wang, Y. et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer's disease model. Cell 160, 1061–1071 (2015).
Saresella, M. et al. A complex proinflammatory role for peripheral monocytes in Alzheimer's disease. J. Alzheimers Dis. 38, 403–413 (2014).
Naert, G. & Rivest, S. A deficiency in CCR2+ monocytes: the hidden side of Alzheimer's disease. J. Mol. Cell. Biol. 5, 284–293 (2013).
Wolf, Y., Yona, S., Kim, K.-W. & Jung, S. Microglia, seen from the CX3CR1 angle. Front. Cell. Neurosci. 7, 26 (2013).
Cardona, A. E. et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 9, 917–924 (2006).
Fuhrmann, M. et al. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat. Neurosci. 13, 411–413 (2010).
Lee, S. et al. CX3CR1 deficiency alters microglial activation and reduces beta-amyloid deposition in two Alzheimer's disease mouse models. Am. J. Pathol. 177, 2549–2562 (2010). This paper was the first to support the role of CX 3 CR1 as a drug target in Alzheimer disease.
Liu, Z., Condello, C., Schain, A., Harb, R. & Grutzendler, J. CX3CR1 in microglia regulates brain amyloid deposition through selective protofibrillar amyloid-β phagocytosis. J. Neurosci. 30, 17091–17101 (2010).
McLarnon, J. G., Ryu, J. K., Walker, D. G. & Choi, H. B. Upregulated expression of purinergic P2X7 receptor in Alzheimer disease and amyloid-beta peptide-treated microglia and in peptide-injected rat hippocampus. J. Neuropathol. Exp. Neurol. 65, 1090–1097 (2006).
Parvathenani, L. K. et al. P2X7 mediates superoxide production in primary microglia and is up-regulated in a transgenic mouse model of Alzheimer's disease. J. Biol. Chem. 278, 13309–13317 (2003).
Sanz, J. M. et al. Possible protective role of the 489C>T P2X7R polymorphism in Alzheimer's disease. Exp. Gerontol. 60, 117–119 (2014).
Sanz, J. M. et al. Activation of microglia by amyloidβ requires P2X7 receptor expression. J. Immunol. 182, 4378–4385 (2009).
Ni, J., Wang, P., Zhang, J., Chen, W. & Gu, L. Silencing of the P2X7 receptor enhances amyloid-β phagocytosis by microglia. Biochem. Biophys. Res. Commun. 434, 363–369 (2013).
Diaz-Hernandez, J. I. et al. In vivo P2X7 inhibition reduces amyloid plaques in Alzheimer's disease through GSK3β and secretases. Neurobiol. Aging 33, 1816–1828 (2012).
Clark, A. K., Gentry, C., Bradbury, E. J., McMahon, S. B. & Malcangio, M. Role of spinal microglia in rat models of peripheral nerve injury and inflammation. Eur. J. Pain Lond. Engl. 11, 223–230 (2007).
Tsuda, M. et al. P2X4 receptors induced in spinal microglia gate tactile allodynia after nerve injury. Nature 424, 778–783 (2003). A landmark study providing evidence for P2X4 in spinal cord microglia as a drug target in neuropathic pain.
Schäfers, M., Svensson, C. I., Sommer, C. & Sorkin, L. S. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J. Neurosci. 23, 2517–2521 (2003).
Chu, Y.-X., Zhang, Y., Zhang, Y.-Q. & Zhao, Z.-Q. Involvement of microglial P2X7 receptors and downstream signaling pathways in long-term potentiation of spinal nociceptive responses. Brain. Behav. Immun. 24, 1176–1189 (2010).
Clark, A. K. et al. P2X7-dependent release of interleukin-1β and nociception in the spinal cord following lipopolysaccharide. J. Neurosci. 30, 573–582 (2010).
Zhou, D., Chen, M.-L., Zhang, Y.-Q. & Zhao, Z.-Q. Involvement of spinal microglial P2X7 receptor in generation of tolerance to morphine analgesia in rats. J. Neurosci. 30, 8042–8047 (2010).
He, W.-J. et al. Spinal P2X7 receptor mediates microglia activation-induced neuropathic pain in the sciatic nerve injury rat model. Behav. Brain Res. 226, 163–170 (2012).
Clark, A. K., Grist, J., Al-Kashi, A., Perretti, M. & Malcangio, M. Spinal cathepsin S and fractalkine contribute to chronic pain in the collagen-induced arthritis model. Arthritis Rheum. 64, 2038–2047 (2012).
Clark, A. K., Wodarski, R., Guida, F., Sasso, O. & Malcangio, M. Cathepsin S release from primary cultured microglia is regulated by the P2X7 receptor. Glia 58, 1710–1726 (2010).
Clark, A. K., Yip, P. K. & Malcangio, M. The liberation of fractalkine in the dorsal horn requires microglial cathepsin S. J. Neurosci. 29, 6945–6954 (2009).
Jung, H. et al. Visualization of chemokine receptor activation in transgenic mice reveals peripheral activation of CCR2 receptors in states of neuropathic pain. J. Neurosci. 29, 8051–8062 (2009).
Zhang, J. et al. Expression of CCR2 in both resident and bone marrow-derived microglia plays a critical role in neuropathic pain. J. Neurosci. 27, 12396–12406 (2007).
Gao, Y.-J. et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J. Neurosci. 29, 4096–4108 (2009).
Biber, K. & Boddeke, E. Neuronal CC chemokines: the distinct roles of CCL21 and CCL2 in neuropathic pain. Front. Cell. Neurosci. 8, 210 (2014).
Ibrahim, M. M. et al. Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS. Proc. Natl Acad. Sci. USA 100, 10529–10533 (2003).
Malan, T. P. et al. CB2 cannabinoid receptor-mediated peripheral antinociception. Pain 93, 239–245 (2001).
Zhang, J. et al. Induction of CB2 receptor expression in the rat spinal cord of neuropathic but not inflammatory chronic pain models. Eur. J. Neurosci. 17, 2750–2754 (2003).
Romero-Sandoval, E. A., Horvath, R., Landry, R. P. & DeLeo, J. A. Cannabinoid receptor type 2 activation induces a microglial anti-inflammatory phenotype and reduces migration via MKP induction and ERK dephosphorylation. Mol. Pain 5, 25 (2009).
Chahrour, M. & Zoghbi, H. Y. The story of Rett syndrome: from clinic to neurobiology. Neuron 56, 422–437 (2007).
Maezawa, I. & Jin, L.-W. Rett syndrome microglia damage dendrites and synapses by the elevated release of glutamate. J. Neurosci. 30, 5346–5356 (2010).
Maezawa, I., Swanberg, S., Harvey, D., LaSalle, J. M. & Jin, L.-W. Rett syndrome astrocytes are abnormal and spread MeCP2 deficiency through gap junctions. J. Neurosci. 29, 5051–5061 (2009).
Derecki, N. C. et al. Wild-type microglia arrest pathology in a mouse model of Rett syndrome. Nature 484, 105–109 (2012).
Wang, J. et al. Wild-type microglia do not reverse pathology in mouse models of Rett syndrome. Nature 521, E1–E4 (2015).
Barden, N. et al. Analysis of single nucleotide polymorphisms in genes in the chromosome 12Q24.31 region points to P2RX7 as a susceptibility gene to bipolar affective disorder. Am. J. Med. Genet. 141B, 374–382 (2006).
Lucae, S. et al. P2RX7, a gene coding for a purinergic ligand-gated ion channel, is associated with major depressive disorder. Hum. Mol. Genet. 15, 2438–2445 (2006).
Boucher, A. A. et al. Resilience and reduced c-Fos expression in P2X7 receptor knockout mice exposed to repeated forced swim test. Neuroscience 189, 170–177 (2011).
Basso, A. M. et al. Behavioral profile of P2X7 receptor knockout mice in animal models of depression and anxiety: relevance for neuropsychiatric disorders. Behav. Brain Res. 198, 83–90 (2009).
Wilkinson, S. M. et al. The first CNS-active carborane: a novel P2X7 receptor antagonist with antidepressant activity. ACS Chem. Neurosci. 5, 335–339 (2014).
Gibney, S. M. & Drexhage, H. A. Evidence for a dysregulated immune system in the etiology of psychiatric disorders. J. Neuroimmune Pharmacol. 8, 900–920 (2013).
Haarman, B. C. M. B. et al. Neuroinflammation in bipolar disorder — a [11C]-(R)-PK11195 positron emission tomography study. Brain. Behav. Immun. 40, 219–225 (2014).
Bonaccorso, S. et al. Increased depressive ratings in patients with hepatitis C receiving interferon-α-based immunotherapy are related to interferon-α-induced changes in the serotonergic system. J. Clin. Psychopharmacol. 22, 86–90 (2002).
Capuron, L. et al. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol. Psychiatry 54, 906–914 (2003).
Comai, S. et al. Effects of PEG-interferon alpha plus ribavirin on tryptophan metabolism in patients with chronic hepatitis C. Pharmacol. Res. 63, 85–92 (2011).
Carvalho, L. A. et al. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of in patients with melancholic major depressive disorder. Transl. Psychiatry 4, e344 (2014).
Drexhage, R. C. et al. The activation of monocyte and T cell networks in patients with bipolar disorder. Brain. Behav. Immun. 25, 1206–1213 (2011).
Campbell, B. M., Charych, E., Lee, A. W. & Möller, T. Kynurenines in CNS disease: regulation by inflammatory cytokines. Front. Neurosci. 8, 12 (2014).
McGeer, P. L. & McGeer, E. G. Inflammatory processes in amyotrophic lateral sclerosis. Muscle Nerve 26, 459–470 (2002).
Beers, D. R. et al. Wild-type microglia extend survival in PU.1 knockout mice with familial amyotrophic lateral sclerosis. Proc. Natl Acad. Sci. USA 103, 16021–16026 (2006). This study identifies PU.1 as a crucial transcription factor and provides the first data that bone marrow transfer is beneficial in an animal model of ALS.
Koval, E. D. et al. Method for widespread microRNA-155 inhibition prolongs survival in ALS-model mice. Hum. Mol. Genet. 22, 4127–4135 (2013).
Butovsky, O. et al. Modulating inflammatory monocytes with a unique microRNA gene signature ameliorates murine ALS. J. Clin. Invest. 122, 3063–3087 (2012).
Nakada, M., Okada, Y. & Yamashita, J. The role of matrix metalloproteinases in glioma invasion. Front. Biosci. J. Virtual Libr. 8, e261–e269 (2003).
Markovic, D. S. et al. Gliomas induce and exploit microglial MT1-MMP expression for tumor expansion. Proc. Natl Acad. Sci. USA 106, 12530–12535 (2009).
Könnecke, H. & Bechmann, I. The role of microglia and matrix metalloproteinases involvement in neuroinflammation and gliomas. Clin. Dev. Immunol. 2013, 914104 (2013).
Da Fonseca, A. C. C. & Badie, B. Microglia and macrophages in malignant gliomas: recent discoveries and implications for promising therapies. Clin. Dev. Immunol. 2013, 264124 (2013).
Coniglio, S. J. et al. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol. Med. 18, 519–527 (2012).
Kalliomäki, J. et al. A randomized, double-blind, placebo-controlled trial of a chemokine receptor 2 (CCR2) antagonist in posttraumatic neuralgia. Pain 154, 761–767 (2013).
Sorge, R. E. et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat. Med. 18, 595–599 (2012).
Keystone, E. C. et al. Clinical evaluation of the efficacy of the P2X7 purinergic receptor antagonist AZD9056 on the signs and symptoms of rheumatoid arthritis in patients with active disease despite treatment with methotrexate or sulphasalazine. Ann. Rheum. Dis. 71, 1630–1635 (2012).
Stock, T. C. et al. Efficacy and safety of CE-224,535, an antagonist of P2X7 receptor, in treatment of patients with rheumatoid arthritis inadequately controlled by methotrexate. J. Rheumatol. 39, 720–727 (2012).
Ali, Z. et al. Pharmacokinetic and pharmacodynamic profiling of a P2X7 receptor allosteric modulator GSK1482160 in healthy human subjects. Br. J. Clin. Pharmacol. 75, 197–207 (2013).
Lord, B. et al. Pharmacology of a novel central nervous system-penetrant P2X7 antagonist JNJ-42253432. J. Pharmacol. Exp. Ther. 351, 628–641 (2014).
Bhattacharya, A. et al. Pharmacological characterization of a novel centrally permeable P2X7 receptor antagonist: JNJ-47965567. Br. J. Pharmacol. 170, 624–640 (2013).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature 446, 1091–1095 (2007).
Garber, K. Genentech's Alzheimer's antibody trial to study disease prevention. Nat. Biotech. 30, 731–732 (2012).
Spencer, B. & Masliah, E. Immunotherapy for Alzheimer's disease: past, present and future. Front. Aging Neurosci. 6, 114 (2014).
Bohrmann, B. et al. Gantenerumab: a novel human anti-Aβ antibody demonstrates sustained cerebral amyloid-β binding and elicits cell-mediated removal of human amyloid-β. J. Alzheimers Dis. 28, 49–69 (2012).
Ostrowitzki, S. et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch. Neurol. 69, 198–207 (2012).
Wisniewski, T. & Goñi, F. Immunotherapeutic approaches for Alzheimer's disease. Neuron 85, 1162–1176 (2015).
Yang, C. & Xiao, S. New developments of clinical trial in immunotherapy for Alzheimer's disease. Curr. Pharm. Biotechnol. 16, 484–491 (2015).
Raghavendra, V., Tanga, F. & DeLeo, J. A. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J. Pharmacol. Exp. Ther. 306, 624–630 (2003).
Ledeboer, A. et al. Minocycline attenuates mechanical allodynia and proinflammatory cytokine expression in rat models of pain facilitation. Pain 115, 71–83 (2005).
Mika, J., Osikowicz, M., Makuch, W. & Przewlocka, B. Minocycline and pentoxifylline attenuate allodynia and hyperalgesia and potentiate the effects of morphine in rat and mouse models of neuropathic pain. Eur. J. Pharmacol. 560, 142–149 (2007).
Osikowicz, M. et al. Glial inhibitors influence the mRNA and protein levels of mGlu2/3, 5 and 7 receptors and potentiate the analgesic effects of their ligands in a mouse model of neuropathic pain. Pain 147, 175–186 (2009).
Redin, G. S. Antibacterial activity in mice of minocycline, a new tetracycline. Antimicrob. Agents Chemother. 6, 371–376 (1966).
Amin, A. R. et al. A novel mechanism of action of tetracyclines: effects on nitric oxide synthases. Proc. Natl Acad. Sci. USA 93, 14014–14019 (1996).
Chen, M. et al. Minocycline inhibits caspase-1 and caspase-3 expression and delays mortality in a transgenic mouse model of Huntington disease. Nat. Med. 6, 797–801 (2000).
Sadowski, T. & Steinmeyer, J. Minocycline inhibits the production of inducible nitric oxide synthase in articular chondrocytes. J. Rheumatol. 28, 336–340 (2001).
Dunston, C. R., Griffiths, H. R., Lambert, P. A., Staddon, S. & Vernallis, A. B. Proteomic analysis of the anti-inflammatory action of minocycline. Proteomics 11, 42–51 (2011).
Szeto, G. L., Pomerantz, J. L., Graham, D. R. M. & Clements, J. E. Minocycline suppresses activation of nuclear factor of activated T cells 1 (NFAT1) in human CD4+ T cells. J. Biol. Chem. 286, 11275–11282 (2011).
Huang, W. C. et al. Direct protection of cultured neurons from ischemia-like injury by minocycline. Anat. Cell Biol. 43, 325–331 (2010).
Schildknecht, S. et al. Neuroprotection by minocycline caused by direct and specific scavenging of peroxynitrite. J. Biol. Chem. 286, 4991–5002 (2011).
Hashimoto, K. & Ishima, T. A novel target of action of minocycline in NGF-induced neurite outgrowth in PC12 cells: translation initiation [corrected] factor eIF4AI. PLoS ONE 5, e15430 (2010).
Ossola, B. et al. Minocycline protects SH-SY5Y cells from 6-hydroxydopamine by inhibiting both caspase-dependent and -independent programmed cell death. J. Neurosci. Res. 90, 682–690 (2012).
Hughes, E. H. et al. Minocycline delays photoreceptor death in the rds mouse through a microglia-independent mechanism. Exp. Eye Res. 78, 1077–1084 (2004).
Fendrick, S. E., Miller, K. R. & Streit, W. J. Minocycline does not inhibit microglia proliferation or neuronal regeneration in the facial nucleus following crush injury. Neurosci. Lett. 385, 220–223 (2005).
Volonté, C., Apolloni, S., Carrì, M. T. & D'Ambrosi, N. ALS: focus on purinergic signalling. Pharmacol. Ther. 132, 111–122 (2011).
Ji, R.-R. Targeting microglial purinergic signaling to improve morphine analgesia. Pain 150, 377–378 (2010).
Koizumi, S., Ohsawa, K., Inoue, K. & Kohsaka, S. Purinergic receptors in microglia: functional modal shifts of microglia mediated by P2 and P1 receptors. Glia 61, 47–54 (2013).
Crain, J. M., Nikodemova, M. & Watters, J. J. Expression of P2 nucleotide receptors varies with age and sex in murine brain microglia. J. Neuroinflamm. 6, 24 (2009).
Inoue, K. Purinergic systems in microglia. Cell. Mol. Life Sci. 65, 3074–3080 (2008).
Clark, A. K. & Malcangio, M. Fractalkine/CX3CR1 signaling during neuropathic pain. Front. Cell. Neurosci. 8, 121 (2014).
Bhaskar, K. et al. Microglial derived tumor necrosis factor-α drives Alzheimer's disease-related neuronal cell cycle events. Neurobiol. Dis. 62, 273–285 (2014).
Lee, S. et al. Opposing effects of membrane-anchored CX3CL1 on amyloid and tau pathologies via the p38 MAPK pathway. J. Neurosci. 34, 12538–12546 (2014).
Limatola, C. & Ransohoff, R. M. Modulating neurotoxicity through CX3CL1/CX3CR1 signaling. Front. Cell. Neurosci. 8, 229 (2014).
Leist, M. & Hartung, T. Inflammatory findings on species extrapolations: humans are definitely no 70-kg mice. Arch. Toxicol. 87, 563–567 (2013).
Mestas, J. & Hughes, C. C. W. Of mice and not men: differences between mouse and human immunology. J. Immunol. 172, 2731–2738 (2004).
Payne, K. J. & Crooks, G. M. Immune-cell lineage commitment: translation from mice to humans. Immunity 26, 674–677 (2007).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl Acad. Sci. USA 110, 3507–3512 (2013). This paper provides convincing evidence that inflammation is different in mice and humans.
Shay, T. et al. Conservation and divergence in the transcriptional programs of the human and mouse immune systems. Proc. Natl Acad. Sci. USA 110, 2946–2951 (2013).
Smith, A. M. & Dragunow, M. The human side of microglia. Trends Neurosci. 37, 125–135 (2014).
Streit, W. J., Xue, Q.-S., Tischer, J. & Bechmann, I. Microglial pathology. Acta Neuropathol. Commun. 2, 142 (2014). This study brings forward the idea that human microglia become senescent or dysfunctional with ageing, thus rendering the brain vulnerable to the development of age-related neurodegenerative diseases.
Becher, B. & Antel, J. P. Comparison of phenotypic and functional properties of immediately ex vivo and cultured human adult microglia. Glia 18, 1–10 (1996).
Becher, B., Fedorowicz, V. & Antel, J. P. Regulation of CD14 expression on human adult central nervous system-derived microglia. J. Neurosci. Res. 45, 375–381 (1996).
Dick, A. D., Pell, M., Brew, B. J., Foulcher, E. & Sedgwick, J. D. Direct ex vivo flow cytometric analysis of human microglial cell CD4 expression: examination of central nervous system biopsy specimens from HIV-seropositive patients and patients with other neurological disease. AIDS 11, 1699–1708 (1997).
Jack, C. S. et al. TLR signaling tailors innate immune responses in human microglia and astrocytes. J. Immunol. 175, 4320–4330 (2005).
Walter, S. et al. The LPS receptor, CD14, in experimental autoimmune encephalomyelitis and multiple sclerosis. Cell. Physiol. Biochem. 17, 167–172 (2006).
Jiang, Z. et al. CD14 is required for MyD88-independent LPS signaling. Nat. Immunol. 6, 565–570 (2005).
Melief, J. et al. Phenotyping primary human microglia: tight regulation of LPS responsiveness. Glia 60, 1506–1517 (2012). This report provides the first data questioning the general responsiveness of human microglia to lipopolysaccharide.
Schneemann, M. & Schoeden, G. Macrophage biology and immunology: man is not a mouse. J. Leukoc. Biol. 81, 579; discussion 580 (2007).
Landry, R. P., Jacobs, V. L., Romero-Sandoval, E. A. & DeLeo, J. A. Propentofylline, a CNS glial modulator does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages. Exp. Neurol. 234, 340–350 (2012).
Watkins, L. R. et al. Commentary on Landry et al. “Propentofylline, a CNS glial modulator, does not decrease pain in post-herpetic neuralgia patients: in vitro evidence for differential responses in human and rodent microglia and macrophages”. Exp. Neurol. 234, 351–353 (2012).
Perrin, S. Preclinical research: make mouse studies work. Nature 507, 423–425 (2014).
Bishop, N. A., Lu, T. & Yankner, B. A. Neural mechanisms of ageing and cognitive decline. Nature 464, 529–535 (2010).
Norden, D. M. & Godbout, J. P. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathol. Appl. Neurobiol. 39, 19–34 (2013).
Sierra, A., Gottfried-Blackmore, A. C., McEwen, B. S. & Bulloch, K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia 55, 412–424 (2007).
Perry, V. H. & Holmes, C. Microglial priming in neurodegenerative disease. Nat. Rev. Neurol. 10, 217–224 (2014).
Lull, M. E. & Block, M. L. Microglial activation and chronic neurodegeneration. Neurotherapeutics 7, 354–365 (2010).
Henry, C. J., Huang, Y., Wynne, A. M. & Godbout, J. P. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1β and anti-inflammatory IL-10 cytokines. Brain. Behav. Immun. 23, 309–317 (2009).
Fenn, A. M., Henry, C. J., Huang, Y., Dugan, A. & Godbout, J. P. Lipopolysaccharide-induced interleukin (IL)-4 receptor-α expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain. Behav. Immun. 26, 766–777 (2012).
Perry, V. H., Cunningham, C. & Holmes, C. Systemic infections and inflammation affect chronic neurodegeneration. Nat. Rev. Immunol. 7, 161–167 (2007).
Streit, W. J. & Xue, Q.-S. Human CNS immune senescence and neurodegeneration. Curr. Opin. Immunol. 29, 93–96 (2014).
Streit, W. J. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 29, 506–510 (2006).
Calado, R. T. & Dumitriu, B. Telomere dynamics in mice and humans. Semin. Hematol. 50, 165–174 (2013).
Hayflick, L. The limited in vitro life time of human diploid cell strains. Exp. Cell Res. 37, 614–636 (1965).
Allsopp, R. C. et al. Telomere shortening is associated with cell division in vitro and in vivo. Exp. Cell Res. 220, 194–200 (1995).
Campisi, J. & d' Adda di Fagagna, F. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell. Biol. 8, 729–740 (2007).
Lansdorp, P. M. Telomeres and disease. EMBO J. 28, 2532–2540 (2009).
Flanary, B. E. & Streit, W. J. Progressive telomere shortening occurs in cultured rat microglia, but not astrocytes. Glia 45, 75–88 (2004).
Olah, M. et al. An optimized protocol for the acute isolation of human microglia from autopsy brain samples. Glia 60, 96–111 (2012).
Chiu, I. M. et al. A neurodegeneration-specific gene-expression signature of acutely isolated microglia from an amyotrophic lateral sclerosis mouse model. Cell Rep. 4, 385–401 (2013).
Orre, M. et al. Isolation of glia from Alzheimer's mice reveals inflammation and dysfunction. Neurobiol. Aging 35, 2746–2760 (2014).
Bartlett, R., Stokes, L. & Sluyter, R. The P2X7 receptor channel: recent developments and the use of P2X7 antagonists in models of disease. Pharmacol. Rev. 66, 638–675 (2014).
Donnelly-Roberts, D. L., Namovic, M. T., Han, P. & Jarvis, M. F. Mammalian P2X7 receptor pharmacology: comparison of recombinant mouse, rat and human P2X7 receptors. Br. J. Pharmacol. 157, 1203–1214 (2009).
Sluyter, R. & Stokes, L. Significance of P2X7 receptor variants to human health and disease. Recent Pat. DNA Gene Seq. 5, 41–54 (2011).
Yiangou, Y. et al. COX-2, CB2 and P2X7-immunoreactivities are increased in activated microglial cells/macrophages of multiple sclerosis and amyotrophic lateral sclerosis spinal cord. BMC Neurol. 6, 12 (2006).
Grygorowicz, T., Sulejczak, D. & Struzynska, L. Expression of purinergic P2X7 receptor in rat brain during the symptomatic phase of experimental autoimmune encephalomyelitis and after recovery of neurological deficits. Acta Neurobiol. Exp. (Wars) 71, 65–73 (2011).
Witting, A. et al. Experimental autoimmune encephalomyelitis disrupts endocannabinoid-mediated neuroprotection. Proc. Natl Acad. Sci. USA 103, 6362–6367 (2006).
Caragnano, M. et al. Monocytes P2X7 purinergic receptor is modulated by glatiramer acetate in multiple sclerosis. J. Neuroimmunol. 245, 93–97 (2012).
Sigurdsson, E. M., Scholtzova, H., Mehta, P. D., Frangione, B. & Wisniewski, T. Immunization with a nontoxic/nonfibrillar amyloid-β homologous peptide reduces Alzheimer's disease-associated pathology in transgenic mice. Am. J. Pathol. 159, 439–447 (2001).
Kellner, A. et al. Autoantibodies against β-amyloid are common in Alzheimer's disease and help control plaque burden. Ann. Neurol. 65, 24–31 (2009).
Zotova, E. et al. Inflammatory components in human Alzheimer's disease and after active amyloid-β42 immunization. Brain J. Neurol. 136, 2677–2696 (2013).
Zotova, E. et al. Microglial alterations in human Alzheimer's disease following Aβ42 immunization. Neuropathol. Appl. Neurobiol. 37, 513–524 (2011).
Walker, D. G. et al. Association of CD33 polymorphism rs3865444 with Alzheimer's disease pathology and CD33 expression in human cerebral cortex. Neurobiol. Aging 36, 517–582 (2015).
Frank, S. et al. TREM2 is upregulated in amyloid plaque-associated microglia in aged APP23 transgenic mice. Glia 56, 1438–1447 (2008).
Schmid, C. D. et al. Heterogeneous expression of the triggering receptor expressed on myeloid cells-2 on adult murine microglia. J. Neurochem. 83, 1309–1320 (2002).
Lucin, K. M. et al. Microglial beclin 1 regulates retromer trafficking and phagocytosis and is impaired in Alzheimer's disease. Neuron 79, 873–886 (2013).
Lue, L.-F. et al. TREM2 protein expression changes correlate with Alzheimer's disease neurodegenerative pathologies in post-mortem temporal cortices. Brain Pathol. 25, 469–480 (2014).
El Khoury, J. et al. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 13, 432–438 (2007).
Naert, G. & Rivest, S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer's disease. J. Neurosci. 31, 6208–6220 (2011).
Liu, Y. et al. IKKβ deficiency in myeloid cells ameliorates Alzheimer's disease-related symptoms and pathology. J. Neurosci. 34, 12982–12999 (2014).
Naert, G. & Rivest, S. Age-related changes in synaptic markers and monocyte subsets link the cognitive decline of APPSwe/PS1 mice. Front. Cell. Neurosci. 6, 51 (2012).
Biber, K. et al. Neuronal CCL21 up-regulates microglia P2X4 expression and initiates neuropathic pain development. EMBO J. 30, 1864–1873 (2011).
Staniland, A. A. et al. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J. Neurochem. 114, 1143–1157 (2010).
Abbadie, C. et al. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc. Natl Acad. Sci. USA 100, 7947–7952 (2003).
Padi, S. S. V. et al. Attenuation of rodent neuropathic pain by an orally active peptide, RAP-103, which potently blocks CCR2- and CCR5-mediated monocyte chemotaxis and inflammation. Pain 153, 95–106 (2012).
Old, E. A. et al. Monocytes expressing CX3CR1 orchestrate the development of vincristine-induced pain. J. Clin. Invest. 124, 2023–2036 (2014).
Corona, A. W. et al. Indoleamine 2,3-dioxygenase inhibition attenuates lipopolysaccharide induced persistent microglial activation and depressive-like complications in fractalkine receptor (CX3CR1)-deficient mice. Brain. Behav. Immun. 31, 134–142 (2013).
O'Connor, J. C. et al. Interferon-γ and tumor necrosis factor-α mediate the upregulation of indoleamine 2,3-dioxygenase and the induction of depressive-like behavior in mice in response to bacillus Calmette-Guérin. J. Neurosci. 29, 4200–4209 (2009).
Busse, M. et al. Decreased quinolinic acid in the hippocampus of depressive patients: evidence for local anti-inflammatory and neuroprotective responses? Eur. Arch. Psychiatry Clin. Neurosci. 265, 321–329 (2015).
Gos, T. et al. Reduced microglial immunoreactivity for endogenous NMDA receptor agonist quinolinic acid in the hippocampus of schizophrenia patients. Brain. Behav. Immun. 41, 59–64 (2014).
Wang, H. et al. S100B promotes glioma growth through chemoattraction of myeloid-derived macrophages. Clin. Cancer Res. 19, 3764–3775 (2013).
Xu, S. et al. Effect of miR-142-3p on the M2 macrophage and therapeutic efficacy against murine glioblastoma. J. Natl. Cancer Inst. 106 (2014).
Panza, F. et al. Efficacy and safety studies of gantenerumab in patients with Alzheimer's disease. Expert Rev. Neurother. 14, 973–986 (2014).
Acknowledgements
The authors apologize to all those colleagues whose work was discussed without proper citation owing to space constraints. M.P. receives support from the Federal Ministry of Education and Research (BMBF)-funded competence network of multiple sclerosis (KKNMS), the Hertie Foundation (GHST), the Sobek Foundation, the Fritz Thyssen Foundation and the German Research Foundation (DFG; grants SFB 992 and FOR 1336). K.B. receives support from the BMBF-funded competence network of neurodegenerative disease (KNDD), the BMBF project ReelinSys and the DFG (grants BI 668/5-1 and BI 668/2-2 (FOR 1336)).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Surveillant microglia
-
Microglia in the healthy brain that constantly screen their microenvironment for potential disturbances in homeostasis or damage.
- Sensome
-
A complex array of numerous receptors and molecular pattern recognition structures that enable microglia to monitor their surroundings.
- Microgliopathies
-
Brain diseases that are caused by mutated, dysfunctional microglia.
- Disease-specific phenotype
-
A cellular state that is specific for a certain disease or even for a specific phase of disease.
- Perivascular macrophages
-
Myeloid cells of the perivascular spaces. These cells do not reside in the brain parenchyma.
- Telomere
-
The end of a chromosome that is important for cellular ageing processes.
Rights and permissions
About this article
Cite this article
Biber, K., Möller, T., Boddeke, E. et al. Central nervous system myeloid cells as drug targets: current status and translational challenges. Nat Rev Drug Discov 15, 110–124 (2016). https://doi.org/10.1038/nrd.2015.14
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd.2015.14
This article is cited by
-
Lipofuscin-like autofluorescence within microglia and its impact on studying microglial engulfment
Nature Communications (2023)
-
Morphofunctional Features of Microglial Cells during the Administration of Orexin A
Bulletin of Experimental Biology and Medicine (2023)
-
Opposing effects of apoE2 and apoE4 on microglial activation and lipid metabolism in response to demyelination
Molecular Neurodegeneration (2022)
-
Microglia and monocytes in inflammatory CNS disease: integrating phenotype and function
Acta Neuropathologica (2022)
-
Astrocyte-derived TNF and glutamate critically modulate microglia activation by methamphetamine
Neuropsychopharmacology (2021)