Key Points
-
Muscle wasting is a debilitating condition that develops with ageing and more rapidly with inactivity (bed rest) and in various systemic diseases (for example, cancer, renal failure, chronic obstructive pulmonary disease, sepsis, HIV and trauma). Fibre atrophy primarily results from an acceleration of protein degradation, often combined with reduced protein synthesis. Treatments that prevent this activation of proteolysis or increase protein synthesis offer considerable promise to combat this debilitating process.
-
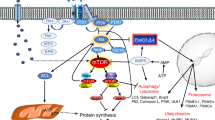
Various types of rapid muscle wasting develop through a common transcriptional programme involving the induction of a set of atrophy-related genes (atrogenes) by forkhead box protein O (FOXO) transcription factors and reduced signalling by the PI3K–AKT–mTOR pathway.
-
The resulting muscle weakness is a consequence of the degradation of myofibrils, which is catalysed by ubiquitin ligases that target different components of the contractile apparatus for proteasomal degradation. By contrast, the loss of endurance results from the breakdown of mitochondria via autophagy.
-
Myostatin, an autocrine inhibitor of normal muscle growth, and its circulating homologue activin A, also trigger muscle protein loss in various catabolic states via the activation of SMAD2 and SMAD3, which function together with FOXO transcription factors.
-
Antibodies against myostatin or activin A, or agents that block their receptor — activin A receptor, type IIB (ActRIIB) — in muscle could be a promising approach to combat muscle loss caused by cancer-associated cachexia, renal failure and ageing. Indeed, these treatments helped to preserve muscle and prolong longevity in tumour-bearing mice, and several of these treatments are currently in clinical trials.
-
Glucocorticoids and various circulating inflammatory mediators, such as tumour necrosis factor-α (TNFα) and interleukin-6 (IL-6), have also been implicated in excessive muscle proteolysis in cachexia, but their roles in different catabolic states remain uncertain and controversial.
-
Recent studies have increased our understanding of the biochemical mechanisms of atrophy and have identified many intracellular proteins that are crucial in muscle wasting (for example, SMADs, tripartate motif-containing protein 32 (TRIM32), nuclear factor-κB (NF-κB)) or that combat this process (for example, peroxisome proliferator-activated receptor-γ coactivator 1α (PGC1α), sirtuin1 (SIRT1) and JUNB). Their manipulation by small molecules offers many opportunities for the rational design of new treatments for this condition.
Abstract
Atrophy occurs in specific muscles with inactivity (for example, during plaster cast immobilization) or denervation (for example, in patients with spinal cord injuries). Muscle wasting occurs systemically in older people (a condition known as sarcopenia); as a physiological response to fasting or malnutrition; and in many diseases, including chronic obstructive pulmonary disorder, cancer-associated cachexia, diabetes, renal failure, cardiac failure, Cushing syndrome, sepsis, burns and trauma. The rapid loss of muscle mass and strength primarily results from excessive protein breakdown, which is often accompanied by reduced protein synthesis. This loss of muscle function can lead to reduced quality of life, increased morbidity and mortality. Exercise is the only accepted approach to prevent or slow atrophy. However, several promising therapeutic agents are in development, and major advances in our understanding of the cellular mechanisms that regulate the protein balance in muscle include the identification of several cytokines, particularly myostatin, and a common transcriptional programme that promotes muscle wasting. Here, we discuss these new insights and the rationally designed therapies that are emerging to combat muscle wasting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jackman, R. W. & Kandarian, S. C. The molecular basis of skeletal muscle atrophy. Am. J. Physiol. Cell Physiol. 287, C834–C843 (2004).
Lecker, S. H., Goldberg, A. L. & Mitch, W. E. Protein degradation by the ubiquitin-proteasome pathway in normal and disease states. J. Am. Soc. Nephrol. 17, 1807–1819 (2006).
Zhou, X. et al. Reversal of cancer cachexia and muscle wasting by ActRIIB antagonism leads to prolonged survival. Cell 142, 531–543 (2010). This article demonstrates that targeting of the myostatin–activin pathway is a promising option in reducing cachexia and its associated morbidity.
Mitch, W. E. & Goldberg, A. L. Mechanisms of muscle wasting. The role of the ubiquitin-proteasome pathway. N. Engl. J. Med. 335, 1897–1905 (1996). This review first established the importance of excessive proteolysis by the UPS in diverse disease.
Lecker, S. H., Solomon, V., Mitch, W. E. & Goldberg, A. L. Muscle protein breakdown and the critical role of the ubiquitin-proteasome pathway in normal and disease states. J. Nutr. 129, 227S–237S (1999).
Jagoe, R. T., Lecker, S. H., Gomes, M. & Goldberg, A. L. Patterns of gene expression in atrophying skeletal muscles: response to food deprivation. FASEB J. 16, 1697–1712 (2002).
Lecker, S. H. et al. Multiple types of skeletal muscle atrophy involve a common program of changes in gene expression. FASEB J. 18, 39–51 (2004).
Gomes, M. D., Lecker, S. H., Jagoe, R. T., Navon, A. & Goldberg, A. L. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc. Natl Acad. Sci. USA 98, 14440–14445 (2001).
Sacheck, J. M. et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 21, 140–155 (2007).
Zhao, J. et al. FoxO3 coordinately activates protein degradation by the autophagic/lysosomal and proteasomal pathways in atrophying muscle cells. Cell Metab. 6, 472–483 (2007).
Mammucari, C. et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab. 6, 458–471 (2007).
Bodine, S. C. et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science 294, 1704–1708 (2001). References 6–12 identify and define atrophy-related genes.
Cohen, S. et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J. Cell Biol. 185, 1083–1095 (2009).
Cohen, S., Zhai, B., Gygi, S. P. & Goldberg, A. L. Ubiquitylation by Trim32 causes coupled loss of desmin, Z-bands, and thin filaments in muscle atrophy. J. Cell Biol. 198, 575–589 (2012). References 13 and 14 describe the mechanism for myofibril disassembly during muscle atrophy.
Clarke, B. A. et al. The E3 Ligase MuRF1 degrades myosin heavy chain protein in dexamethasone-treated skeletal muscle. Cell Metab. 6, 376–385 (2007).
Lagirand-Cantaloube, J. et al. The initiation factor eIF3-f is a major target for atrogin1/MAFbx function in skeletal muscle atrophy. EMBO J. 27, 1266–1276 (2008).
Latres, E. et al. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J. Biol. Chem. 280, 2737–2744 (2005).
Bonaldo, P. & Sandri, M. Cellular and molecular mechanisms of muscle atrophy. Dis. Model. Mech. 6, 25–39 (2013).
Schiaffino, S. & Mammucari, C. Regulation of skeletal muscle growth by the IGF1-Akt/PKB pathway: insights from genetic models. Skelet. Muscle 1, 4 (2011).
Glass, D. J. Skeletal muscle hypertrophy and atrophy signaling pathways. Int. J. Biochem. Cell Biol. 37, 1974–1984 (2005).
Sandri, M. et al. FoxO transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell 117, 399–412 (2004).
Sartori, R. et al. Smad2 and 3 transcription factors control muscle mass in adulthood. American journal of physiology. Cell Physiol. 296, C1248–C1257 (2009). This study established the critical role of FOXO proteins in causing atrophy.
Menconi, M. et al. Role of glucocorticoids in the molecular regulation of muscle wasting. Crit. Care Med. 35, S602–S608 (2007).
Cai, D. et al. IKKβ/NF-κB activation causes severe muscle wasting in mice. Cell 119, 285–298 (2004).
Mourkioti, F. et al. Targeted ablation of IKK2 improves skeletal muscle strength, maintains mass, and promotes regeneration. J. Clin. Invest. 116, 2945–2954 (2006).
Allen, D. L. & Unterman, T. G. Regulation of myostatin expression and myoblast differentiation by FoxO and SMAD transcription factors. Am.J. Physiol. Cell Physiol. 292, C188–C199 (2007).
Glass, D. J. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Curr. Top. Microbiol. Immunol. 346, 267–278 (2010).
Scott, R. C., Schuldiner, O. & Neufeld, T. P. Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev. Cell 7, 167–178 (2004).
Shimizu, N. et al. Crosstalk between glucocorticoid receptor and nutritional sensor mTOR in skeletal muscle. Cell Metab. 13, 170–182 (2011).
Sacheck, J. M., Ohtsuka, A., McLary, S. C. & Goldberg, A. L. IGF-I stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 287, E591–E601 (2004).
Lai, K. M. et al. Conditional activation of akt in adult skeletal muscle induces rapid hypertrophy. Mol. Cell. Biol. 24, 9295–9304 (2004).
Han, H. Q., Zhou, X., Mitch, W. E. & Goldberg, A. L. Myostatin/activin pathway antagonism: Molecular basis and therapeutic potential. Int. J. Biochem. Cell Biol. 45, 2333–2347 (2013).
Trendelenburg, A. U. et al. Myostatin reduces Akt/TORC1/p70S6K signaling, inhibiting myoblast differentiation and myotube size. Am. J. Physiol. Cell Physiol. 296, C1258–C1270 (2009).
Raffaello, A. et al. JunB transcription factor maintains skeletal muscle mass and promotes hypertrophy. J. Cell Biol. 191, 101–113 (2010). This study demonstrates the crucial role of JUNB in maintaining muscle mass.
Piechaczyk, M. & Farras, R. Regulation and function of JunB in cell proliferation. Biochem. Soc. Trans. 36, 864–867 (2008).
Sandri, M. et al. PGC-1α protects skeletal muscle from atrophy by suppressing FoxO3 action and atrophy-specific gene transcription. Proc. Natl Acad. Sci. USA 103, 16260–16265 (2006).
Brault, J. J., Jespersen, J. G. & Goldberg, A. L. Peroxisome proliferator-activated receptor γ coactivator 1α or 1β overexpression inhibits muscle protein degradation, induction of ubiquitin ligases, and disuse atrophy. J. Biol. Chem. 285, 19460–19471 (2010).
Ruas, J. L. et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell 151, 1319–1331 (2012).
Handschin, C. & Spiegelman, B. M. The role of exercise and PGC1α in inflammation and chronic disease. Nature 454, 463–469 (2008).
Vega, R. B., Huss, J. M. & Kelly, D. P. The coactivator PGC-1 cooperates with peroxisome proliferator-activated receptor α in transcriptional control of nuclear genes encoding mitochondrial fatty acid oxidation enzymes. Mol. Cell. Biol. 20, 1868–1876 (2000).
Yamazaki, Y. et al. The cathepsin L gene is a direct target of FOXO1 in skeletal muscle. Biochem. J. 427, 171–178 (2010).
Waddell, D. S. et al. The glucocorticoid receptor and FOXO1 synergistically activate the skeletal muscle atrophy-associated MuRF1 gene. Am. J. Physiol. Endocrinol. Metab. 295, E785–E797 (2008).
Smith, I. J. et al. Sepsis increases the expression and activity of the transcription factor Forkhead Box O1 (FOXO1) in skeletal muscle by a glucocorticoid-dependent mechanism. Int. J. Biochem. Cell Biol. 42, 701–711 (2010).
Wei, B. et al. MST1, a key player, in enhancing fast skeletal muscle atrophy. BMC Biol. 11, 12 (2013).
Greer, E. L. et al. An AMPK-FOXO pathway mediates longevity induced by a novel method of dietary restriction in C. elegans. Curr. Biol. 17, 1646–1656 (2007).
Greer, E. L. et al. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J. Biol. Chem. 282, 30107–30119 (2007).
Lee, D. & Goldberg, A. L. SIRT1 by blocking the activities of FoxO1 and 3 inhibits muscle atrophy and promotes muscle growth. J. Biol. Chem. 288, 30515–30526 (2013).
Bertaggia, E., Coletto, L. & Sandri, M. Posttranslational modifications control FoxO3 activity during denervation. American journal of physiology. Cell Physiol. 302, C587–C596 (2012).
Hunter, R. B. & Kandarian, S. C. Disruption of either the Nfkb1 or the Bcl3 gene inhibits skeletal muscle atrophy. J. Clin. Invest. 114, 1504–1511 (2004).
Reed, S. A., Senf, S. M., Cornwell, E. W., Kandarian, S. C. & Judge, A. R. Inhibition of IκB kinase α (IKKα) or IKKβ (IKKβ) plus forkhead box O (Foxo) abolishes skeletal muscle atrophy. Biochem. Biophys. Res. Commun. 405, 491–496 (2011).
Guttridge, D. C., Mayo, M. W., Madrid, L. V., Wang, C. Y. & Baldwin, A. S. Jr. NF-κB-induced loss of MyoD messenger RNA: possible role in muscle decay and cachexia. Science 289, 2363–2366 (2000).
Sriram, S. et al. Modulation of reactive oxygen species in skeletal muscle by myostatin is mediated through NF-κB. Aging Cell 10, 931–948 (2011).
Paul, P. K. et al. The E3 ubiquitin ligase TRAF6 intercedes in starvation-induced skeletal muscle atrophy through multiple mechanisms. Mol. Cell. Biol. 32, 1248–1259 (2012).
Reid, M. B. & Li, Y. P. Tumor necrosis factor-α and muscle wasting: a cellular perspective. Respir. Res. 2, 269–272 (2001).
Yamaki, T. et al. Rel A/p65 is required for cytokine-induced myotube atrophy. Am. J. Physiol. Cell Physiol. 303, C135–C142 (2012).
Dogra, C. et al. TNF-related weak inducer of apoptosis (TWEAK) is a potent skeletal muscle-wasting cytokine. FASEB J. 21, 1857–1869 (2007).
Mittal, A. et al. The TWEAK-Fn14 system is a critical regulator of denervation-induced skeletal muscle atrophy in mice. J. Cell Biol. 188, 833–849 (2010).
Burke, J. R. et al. BMS-345541 is a highly selective inhibitor of IκB kinase that binds at an allosteric site of the enzyme and blocks NF-κB-dependent transcription in mice. J. Biol. Chem. 278, 1450–1456 (2003).
Sharma, V., Lansdell, T. A., Peddibhotla, S. & Tepe, J. J. Sensitization of tumor cells toward chemotherapy: enhancing the efficacy of camptothecin with imidazolines. Chem. Biol. 11, 1689–1699 (2004).
Dewey, A., Baughan, C., Dean, T., Higgins, B. & Johnson, I. Eicosapentaenoic acid (EPA, an omega-3 fatty acid from fish oils) for the treatment of cancer cachexia. Cochrane Database Syst Rev, CD004597 (2007).
Karin, M., Yamamoto, Y. & Wang, Q. M. The IKK NF-κB system: a treasure trove for drug development. Nature Rev. Drug Discov. 3, 17–26 (2004).
McCroskery, S., Thomas, M., Maxwell, L., Sharma, M. & Kambadur, R. Myostatin negatively regulates satellite cell activation and self-renewal. J. Cell Biol. 162, 1135–1147 (2003).
Wagner, K. R., Liu, X., Chang, X. & Allen, R. E. Muscle regeneration in the prolonged absence of myostatin. Proc. Natl Acad. Sci. USA 102, 2519–2524 (2005).
Hittel, D. S. et al. Myostatin decreases with aerobic exercise and associates with insulin resistance. Med. Sci. Sports Exerc. 42, 2023–2029 (2010).
Watts, R., McAinch, A. J., Dixon, J. B., O'Brien, P. E. & Cameron-Smith, D. Increased Smad signaling and reduced MRF expression in skeletal muscle from obese subjects. Obesity (Silver Spring) 21, 525–528 (2013).
Lang, C. H., Silvis, C., Nystrom, G. & Frost, R. A. Regulation of myostatin by glucocorticoids after thermal injury. FASEB J. 15, 1807–1809 (2001).
Schakman, O., Gilson, H. & Thissen, J. P. Mechanisms of glucocorticoid-induced myopathy. J. Endocrinol. 197, 1–10 (2008).
Lach-Trifilieff, E. et al. An antibody blocking activin type II receptors induces strong skeletal muscle hypertrophy and protects from atrophy. Mol. Cell. Biol. 34, 606–618 (2014).
Lee, S. J. & McPherron, A. C. Regulation of myostatin activity and muscle growth. Proc. Natl Acad. Sci. USA 98, 9306–9311 (2001).
Lipina, C., Kendall, H., McPherron, A. C., Taylor, P. M. & Hundal, H. S. Mechanisms involved in the enhancement of mammalian target of rapamycin signalling and hypertrophy in skeletal muscle of myostatin-deficient mice. FEBS Lett. 584, 2403–2408 (2010).
Sartori, R. et al. BMP signaling controls muscle mass. Nature Genet. 45, 1309–1318 (2013).
Shan, T., Liang, X., Bi, P. & Kuang, S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 27, 1981–1989 (2013).
Fielitz, J. et al. Myosin accumulation and striated muscle myopathy result from the loss of muscle RING finger 1 and 3. J. Clin. Invest. 117, 2486–2495 (2007).
Kudryashova, E., Kudryashov, D., Kramerova, I. & Spencer, M. J. Trim32 is a ubiquitin ligase mutated in limb girdle muscular dystrophy type 2H that binds to skeletal muscle myosin and ubiquitinates actin. J. Mol. Biol. 354, 413–424 (2005).
Frosk, P. et al. Limb-girdle muscular dystrophy type 2H associated with mutation in TRIM32, a putative E3-ubiquitin-ligase gene. Am. J. Hum. Genet. 70, 663–672 (2002).
Kudryashova, E., Wu, J., Havton, L. A. & Spencer, M. J. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Hum. Mol. Genet. 18, 1353–1367 (2009).
Kudryashova, E., Kramerova, I. & Spencer, M. J. Satellite cell senescence underlies myopathy in a mouse model of limb-girdle muscular dystrophy 2H. J. Clin. Invest. 122, 1764–1776 (2012).
Cohen, S., Lee, D., Zhai, B., Gygi, S. P. & Goldberg, A. L. Trim32 reduces PI3K-Akt-FoxO signaling in muscle atrophy by promoting plakoglobin-PI3K dissociation. J. Cell Biol. 204, 747–758 (2014).
Sandri, M. Signaling in muscle atrophy and hypertrophy. Physiology 23, 160–170 (2008).
Rabinovich, E., Kerem, A., Frohlich, K. U., Diamant, N. & Bar-Nun, S. AAA-ATPase p97/Cdc48p, a cytosolic chaperone required for endoplasmic reticulum-associated protein degradation. Mol. Cell. Biol. 22, 626–634 (2002).
Piccirillo, R. & Goldberg, A. L. The p97/VCP ATPase is critical in muscle atrophy and the accelerated degradation of muscle proteins. EMBO J. 31, 3334–3350 (2012).
Chou, T. F. et al. Reversible inhibitor of p97, DBeQ, impairs both ubiquitin-dependent and autophagic protein clearance pathways. Proc. Natl Acad. Sci. USA 108, 4834–4839 (2011).
Magnaghi, P. et al. Covalent and allosteric inhibitors of the ATPase VCP/p97 induce cancer cell death. Nature Chem. Biol. 9, U548–U544 (2013).
Watts, G. D. et al. Inclusion body myopathy associated with Paget disease of bone and frontotemporal dementia is caused by mutant valosin-containing protein. Nature Genet. 36, 377–381 (2004).
Johnson, J. O. et al. Exome sequencing reveals VCP mutations as a cause of familial ALS. Neuron 68, 857–864 (2010).
Goldberg, A. L. Development of proteasome inhibitors as research tools and cancer drugs. J. Cell Biol. 199, 583–588 (2012).
Jamart, C., Raymackers, J. M., Li An, G., Deldicque, L. & Francaux, M. Prevention of muscle disuse atrophy by MG132 proteasome inhibitor. Muscle Nerve 43, 708–716 (2011).
Caron, A. Z. et al. The proteasome inhibitor MG132 reduces immobilization-induced skeletal muscle atrophy in mice. BMC Musculoskelet. Disord. 12, 185 (2011).
Supinski, G. S., Vanags, J. & Callahan, L. A. Effect of proteasome inhibitors on endotoxin-induced diaphragm dysfunction. Am. J. Physiol. Lung Cell. Mol. Physiol. 296, L994–L1001 (2009).
Evans, W. J. et al. Cachexia: a new definition. Clin. Nutr. 27, 793–799 (2008).
Baracos, V. E., DeVivo, C., Hoyle, D. H. & Goldberg, A. L. Activation of the ATP-ubiquitin-proteasome pathway in skeletal muscle of cachectic rats bearing a hepatoma. Am. J. Physiol. 268, E996–E1006 (1995).
Bossola, M. et al. Increased muscle proteasome activity correlates with disease severity in gastric cancer patients. Ann. Surg. 237, 384–389 (2003).
Williams, A., Sun, X., Fischer, J. E. & Hasselgren, P. O. The expression of genes in the ubiquitin-proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery 126, 744–749 (1999).
Oliff, A. et al. Tumors secreting human TNF/cachectin induce cachexia in mice. Cell 50, 555–563 (1987).
Bhatnagar, S. et al. Tumor necrosis factor-α regulates distinct molecular pathways and gene networks in cultured skeletal muscle cells. PLoS ONE 5, e13262 (2010).
Costelli, P. et al. Tumor necrosis factor-α mediates changes in tissue protein turnover in a rat cancer cachexia model. J. Clin. Invest. 92, 2783–2789 (1993).
Llovera, M. et al. Anti-TNF treatment reverts increased muscle ubiquitin gene expression in tumour-bearing rats. Biochem. Biophys. Res. Commun. 221, 653–655 (1996).
Kettelhut, I. C. & Goldberg, A. L. Tumor necrosis factor can induce fever in rats without activating protein breakdown in muscle or lipolysis in adipose tissue. J. Clin. Invest. 81, 1384–1389 (1988).
Kettelhut, I. C., Fiers, W. & Goldberg, A. L. The toxic effects of tumor necrosis factor in vivo and their prevention by cyclooxygenase inhibitors. Proc. Natl Acad. Sci. USA 84, 4273–4277 (1987).
Jatoi, A. et al. A placebo-controlled double blind trial of etanercept for the cancer anorexia/weight loss syndrome: results from N00C1 from the North Central Cancer Treatment Group. Cancer 110, 1396–1403 (2007).
Jatoi, A. et al. A placebo-controlled, double-blind trial of infliximab for cancer-associated weight loss in elderly and/or poor performance non-small cell lung cancer patients (N01C9). Lung Cancer 68, 234–239 (2010).
Catalano, M. G. et al. Selective up-regulation of tumor necrosis factor receptor I in tumor-bearing rats with cancer-related cachexia. Int. J. Oncol. 23, 429–436 (2003).
Ebrahimi, B., Tucker, S. L., Li, D., Abbruzzese, J. L. & Kurzrock, R. Cytokines in pancreatic carcinoma: correlation with phenotypic characteristics and prognosis. Cancer 101, 2727–2736 (2004).
Kuroda, K. et al. Interleukin 6 is associated with cachexia in patients with prostate cancer. Urology 69, 113–117 (2007).
Oka, M. et al. Relationship between serum levels of interleukin 6, various disease parameters and malnutrition in patients with esophageal squamous cell carcinoma. Cancer Res. 56, 2776–2780 (1996).
Scott, H. R., McMillan, D. C., Crilly, A., McArdle, C. S. & Milroy, R. The relationship between weight loss and interleukin 6 in non-small-cell lung cancer. Br. J. Cancer 73, 1560–1562 (1996).
Strassmann, G., Fong, M., Kenney, J. S. & Jacob, C. O. Evidence for the involvement of interleukin 6 in experimental cancer cachexia. J. Clin. Invest. 89, 1681–1684 (1992).
Bayliss, T. J., Smith, J. T., Schuster, M., Dragnev, K. H. & Rigas, J. R. A humanized anti-IL-6 antibody (ALD518) in non-small cell lung cancer. Expert Opin. Biol. Ther. 11, 1663–1668 (2011).
Benny Klimek, M. E. et al. Acute inhibition of myostatin-family proteins preserves skeletal muscle in mouse models of cancer cachexia. Biochem. Biophys. Res. Commun. 391, 1548–1554 (2010).
Busquets, S. et al. Myostatin blockage using actRIIB antagonism in mice bearing the Lewis lung carcinoma results in the improvement of muscle wasting and physical performance. J. Cachexia Sarcopenia Muscle 3, 37–43 (2012).
Murphy, K. T. et al. Antibody-directed myostatin inhibition enhances muscle mass and function in tumor-bearing mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R716–R726 (2011). References 109–111 describe several approaches to inhibit myostatin signalling and cachexia in tumour-bearing mice.
Bolton, C. F. et al. Critically ill polyneuropathy: electrophysiological studies and differentiation from Guillain-Barre syndrome. J. Neurol. Neurosurg. Psychiatry 49, 563–573 (1986).
de Letter, M. A. et al. Risk factors for the development of polyneuropathy and myopathy in critically ill patients. Crit. Care Med. 29, 2281–2286 (2001).
Larsson, L. Acute quadriplegic myopathy: an acquired “myosinopathy”. Adv. Exp. Med. Biol. 642, 92–98 (2008).
Hund, E. Myopathy in critically ill patients. Crit. Care Med. 27, 2544–2547 (1999).
Helliwell, T. R. et al. Muscle fibre atrophy in critically ill patients is associated with the loss of myosin filaments and the presence of lysosomal enzymes and ubiquitin. Neuropathol. Appl. Neurobiol. 24, 507–517 (1998).
Levine, S. et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 358, 1327–1335 (2008).
Castillero, E., Alamdari, N., Aversa, Z., Gurav, A. & Hasselgren, P. O. PPARβ/δ regulates glucocorticoid- and sepsis-induced FOXO1 activation and muscle wasting. PLoS ONE 8, e59726 (2013).
Nystrom, G. J. & Lang, C. H. Sepsis and AMPK activation by AICAR differentially regulate FoxO-1, -3 and -4 mRNA in striated muscle. Int. J. Clin. Exp. Med. 1, 50–63 (2008).
Proserpio, V., Fittipaldi, R., Ryall, J. G., Sartorelli, V. & Caretti, G. The methyltransferase SMYD3 mediates the recruitment of transcriptional cofactors at the myostatin and c-Met genes and regulates skeletal muscle atrophy. Genes Dev. 27, 1299–1312 (2013).
Dong, Y., Pan, J. S. & Zhang, L. Myostatin suppression of Akirin1 mediates glucocorticoid-induced satellite cell dysfunction. PLoS ONE 8, e58554 (2013).
Qin, J. et al. Dexamethasone-induced skeletal muscle atrophy was associated with upregulation of myostatin promoter activity. Res. Vet. Sci. 94, 84–89 (2013).
Lang, C. H., Huber, D. & Frost, R. A. Burn-induced increase in atrogin-1 and MuRF-1 in skeletal muscle is glucocorticoid independent but downregulated by IGF-I. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R328–R336 (2007).
Pickering, W. P. et al. Glucocorticoid antagonist RU38486 fails to block acid-induced muscle wasting in vivo or in vitro. Nephrol. Dial. Transplant 18, 1475–1484 (2003).
Smith, I. J., Aversa, Z., Alamdari, N., Petkova, V. & Hasselgren, P. O. Sepsis downregulates myostatin mRNA levels without altering myostatin protein levels in skeletal muscle. J. Cell Biochem. 111, 1059–1073 (2010).
Coats, A. J. Origin of symptoms in patients with cachexia with special reference to weakness and shortness of breath. Int. J. Cardiol. 85, 133–139 (2002).
Workeneh, B. T. & Mitch, W. E. Review of muscle wasting associated with chronic kidney disease. Am. J. Clin. Nutr. 91, 1128S–1132S (2010).
Marquis, K. et al. Midthigh muscle cross-sectional area is a better predictor of mortality than body mass index in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 166, 809–813 (2002).
Swallow, E. B. et al. Quadriceps strength predicts mortality in patients with moderate to severe chronic obstructive pulmonary disease. Thorax 62, 115–120 (2007).
Vestbo, J. et al. Body mass, fat-free body mass, and prognosis in patients with chronic obstructive pulmonary disease from a random population sample: findings from the Copenhagen City Heart Study. Am. J. Respir. Crit. Care Med. 173, 79–83 (2006).
Crul, T. et al. Gene expression profiling in vastus lateralis muscle during an acute exacerbation of COPD. Cell. Physiol. Biochem. 25, 491–500 (2010).
Doucet, M. et al. Atrophy and hypertrophy signalling of the quadriceps and diaphragm in COPD. Thorax 65, 963–970 (2010).
Testelmans, D. et al. Atrophy and hypertrophy signalling in the diaphragm of patients with COPD. Eur. Respir. J. 35, 549–556 (2010).
Thomas, S. S. & Mitch, W. E. Mechanisms stimulating muscle wasting in chronic kidney disease: the roles of the ubiquitin-proteasome system and myostatin. Clin. Exp. Nephrol. 17, 174–182 (2013).
Mitch, W. E. et al. Evaluation of signals activating ubiquitin-proteasome proteolysis in a model of muscle wasting. Am. J. Physiol. 276, C1132–C1138 (1999).
Hu, Z., Wang, H., Lee, I. H., Du, J. & Mitch, W. E. Endogenous glucocorticoids and impaired insulin signaling are both required to stimulate muscle wasting under pathophysiological conditions in mice. J. Clin. Invest. 119, 3059–3069 (2009).
Du, J. et al. Activation of caspase-3 is an initial step triggering accelerated muscle proteolysis in catabolic conditions. J. Clin. Invest. 113, 115–123 (2004).
Workeneh, B. T. et al. Development of a diagnostic method for detecting increased muscle protein degradation in patients with catabolic conditions. J. Am. Soc. Nephrol. 17, 3233–3239 (2006).
Solomon, V., Baracos, V., Sarraf, P. & Goldberg, A. L. Rates of ubiquitin conjugation increase when muscles atrophy, largely through activation of the N-end rule pathway. Proc. Natl Acad. Sci. USA 95, 12602–12607 (1998).
Solomon, V., Lecker, S. H. & Goldberg, A. L. The N-end rule pathway catalyzes a major fraction of the protein degradation in skeletal muscle. J. Biol. Chem. 273, 25216–25222 (1998).
Wang, X. H. et al. Caspase-3 cleaves specific 19 S proteasome subunits in skeletal muscle stimulating proteasome activity. J. Biol. Chem. 285, 21249–21257 (2010).
Plant, P. J., Bain, J. R., Correa, J. E., Woo, M. & Batt, J. Absence of caspase-3 protects against denervation-induced skeletal muscle atrophy. J. Appl. Physiol. 107, 224–234 (2009).
Hu, J. et al. XIAP reduces muscle proteolysis induced by CKD. J. Am. Soc. Nephrol. 21, 1174–1183 (2010).
Demontis, F., Piccirillo, R., Goldberg, A. L. & Perrimon, N. Mechanisms of skeletal muscle aging: insights from Drosophila and mammalian models. Dis. Model. Mech. 6, 1339–1352 (2013).
Lozano, R. et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2095–2128 (2012).
de Man, F. S. et al. Diaphragm muscle fiber weakness in pulmonary hypertension. Am. J. Respir. Crit. Care Med. 183, 1411–1418 (2011).
Mainguy, V. et al. Peripheral muscle dysfunction in idiopathic pulmonary arterial hypertension. Thorax 65, 113–117 (2010).
Batt, J., Shadly Ahmed, S., Correa, J., Bain, A. & Granton, J. Skeletal muscle dysfunction in idiopathic pulmonary arterial hypertension. Am. J. Respir. Cell. Mol. Biol. 50, 74–86 (2013).
Sillau, A. H. & Banchero, N. Effects of hypoxia on capillary density and fiber composition in rat skeletal muscle. Pflugers Arch. 370, 227–232 (1977).
Chaudhary, P. et al. Chronic hypobaric hypoxia mediated skeletal muscle atrophy: role of ubiquitin-proteasome pathway and calpains. Mol. Cell Biochem. 364, 101–113 (2012).
Howald, H. & Hoppeler, H. Performing at extreme altitude: muscle cellular and subcellular adaptations. Eur. J. Appl. Physiol. 90, 360–364 (2003).
Zattara-Hartmann, M. C., Badier, M., Guillot, C., Tomei, C. & Jammes, Y. Maximal force and endurance to fatigue of respiratory and skeletal muscles in chronic hypoxemic patients: the effects of oxygen breathing. Muscle Nerve 18, 495–502 (1995).
von Haehling, S., Lainscak, M., Springer, J. & Anker, S. D. Cardiac cachexia: a systematic overview. Pharmacol. Ther. 121, 227–252 (2009).
Sharma, M. et al. Myostatin, a transforming growth factor-β superfamily member, is expressed in heart muscle and is upregulated in cardiomyocytes after infarct. J. Cell. Physiol. 180, 1–9 (1999).
Heineke, J. et al. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 121, 419–425 (2010).
Loffredo, F. S. et al. Growth differentiation factor 11 is a circulating factor that reverses age-related cardiac hypertrophy. Cell 153, 828–839 (2013).
Muscaritoli, M. et al. Consensus definition of sarcopenia, cachexia and pre-cachexia: joint document elaborated by Special Interest Groups (SIG) “cachexia-anorexia in chronic wasting diseases” and “nutrition in geriatrics”. Clin. Nutr. 29, 154–159 (2010).
Cosqueric, G. et al. Sarcopenia is predictive of nosocomial infection in care of the elderly. Br. J. Nutr. 96, 895–901 (2006).
Keller, J. N., Hanni, K. B. & Markesbery, W. R. Possible involvement of proteasome inhibition in aging: implications for oxidative stress. Mech. Ageing Dev. 113, 61–70 (2000).
Altun, M. et al. Muscle wasting in aged, sarcopenic rats is associated with enhanced activity of the ubiquitin proteasome pathway. J. Biol. Chem. 285, 39597–39608 (2010).
Combaret, L. et al. Skeletal muscle proteolysis in aging. Curr. Opin. Clin. Nutr. Metab. Care 12, 37–41 (2009).
Whitman, S. A., Wacker, M. J., Richmond, S. R. & Godard, M. P. Contributions of the ubiquitin-proteasome pathway and apoptosis to human skeletal muscle wasting with age. Pflugers Arch. 450, 437–446 (2005).
Ibebunjo, C. et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell. Biol. 33, 194–212 (2013). This study identifies the transcriptional changes that occur during sarcopenia.
Deschenes, M. R., Roby, M. A., Eason, M. K. & Harris, M. B. Remodeling of the neuromuscular junction precedes sarcopenia related alterations in myofibers. Exp. Gerontol. 45, 389–393 (2010).
Chai, R. J., Vukovic, J., Dunlop, S., Grounds, M. D. & Shavlakadze, T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS ONE 6, e28090 (2011).
Vermeulen, A. Clinical review 24: Androgens in the aging male. J. Clin. Endocrinol. Metab. 73, 221–224 (1991).
Khosla, S. et al. Relationship of serum sex steroid levels and bone turnover markers with bone mineral density in men and women: a key role for bioavailable estrogen. J. Clin. Endocrinol. Metab. 83, 2266–2274 (1998).
Hermann, M. & Berger, P. Hormonal changes in aging men: a therapeutic indication? Exp. Gerontol. 36, 1075–1082 (2001).
Zadik, Z. et al. The influence of age on the 24-hour integrated concentration of growth hormone in normal individuals. J. Clin. Endocrinol. Metab. 60, 513–516 (1985).
Brill, K. T. et al. Single and combined effects of growth hormone and testosterone administration on measures of body composition, physical performance, mood, sexual function, bone turnover, and muscle gene expression in healthy older men. J. Clin. Endocrinol. Metab. 87, 5649–5657 (2002).
Srinivas-Shankar, U. et al. Effects of testosterone on muscle strength, physical function, body composition, and quality of life in intermediate-frail and frail elderly men: a randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 95, 639–650.
Siriett, V. et al. Prolonged absence of myostatin reduces sarcopenia. J. Cell. Physiol. 209, 866–873 (2006).
Siriett, V. et al. Antagonism of myostatin enhances muscle regeneration during sarcopenia. Mol. Ther. 15, 1463–1470 (2007).
Baccarelli, A. et al. Activin A serum levels and aging of the pituitary-gonadal axis: a cross-sectional study in middle-aged and elderly healthy subjects. Exp. Gerontol. 36, 1403–1412 (2001).
Yarasheski, K. E., Bhasin, S., Sinha-Hikim, I., Pak-Loduca, J. & Gonzalez-Cadavid, N. F. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J. Nutr. Health Aging 6, 343–348 (2002).
LeBrasseur, N. K. et al. Myostatin inhibition enhances the effects of exercise on performance and metabolic outcomes in aged mice. J. Gerontol. A Biol. Sci. Med. Sci. 64, 940–948 (2009).
Murphy, K. T. et al. Antibody-directed myostatin inhibition in 21-mo-old mice reveals novel roles for myostatin signaling in skeletal muscle structure and function. FASEB J. 24, 4433–4442 (2010).
Adams, G. R., Haddad, F., Bodell, P. W., Tran, P. D. & Baldwin, K. M. Combined isometric, concentric, and eccentric resistance exercise prevents unloading-induced muscle atrophy in rats. J. Appl. Physiol. 103, 1644–1654 (2007).
Gielen, S. et al. Exercise training attenuates MuRF-1 expression in the skeletal muscle of patients with chronic heart failure independent of age: the randomized Leipzig Exercise Intervention in Chronic Heart Failure and Aging catabolism study. Circulation 125, 2716–2727 (2012).
Hurst, J. E. & Fitts, R. H. Hindlimb unloading-induced muscle atrophy and loss of function: protective effect of isometric exercise. J. Appl. Physiol. 95, 1405–1417 (2003).
Vissing, K. et al. Effect of resistance exercise contraction mode and protein supplementation on members of the STARS signalling pathway. J. Physiol. 591, 3749–3763 (2013).
Ferrara, N. et al. Exercise training promotes SIRT1 activity in aged rats. Rejuven. Res. 11, 139–150 (2008).
Lin, J. et al. Transcriptional co-activator PGC-1 α drives the formation of slow-twitch muscle fibres. Nature 418, 797–801 (2002).
Arany, Z. et al. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 5, 35–46 (2007).
Shao, D. et al. PGC-1 β-regulated mitochondrial biogenesis and function in myotubes is mediated by NRF-1 and ERR α. Mitochondrion 10, 516–527 (2010).
Wallace, M. A. et al. Striated muscle activator of Rho signalling (STARS) is a PGC-1α/oestrogen-related receptor-α target gene and is upregulated in human skeletal muscle after endurance exercise. J. Physiol. 589, 2027–2039 (2011).
McGee, S. L. & Hargreaves, M. Exercise and myocyte enhancer factor 2 regulation in human skeletal muscle. Diabetes 53, 1208–1214 (2004).
Russell, A. P. PGC-1α and exercise: important partners in combating insulin resistance. Curr. Diabetes Rev. 1, 175–181 (2005).
Arany, Z. et al. The transcriptional coactivator PGC-1β drives the formation of oxidative type IIX fibers in skeletal muscle. Cell Metab. 5, 35–46 (2007).
Wenz, T., Rossi, S. G., Rotundo, R. L., Spiegelman, B. M. & Moraes, C. T. Increased muscle PGC-1α expression protects from sarcopenia and metabolic disease during aging. Proc. Natl Acad. Sci. USA 106, 20405–20410 (2009).
Rera, M. et al. Modulation of longevity and tissue homeostasis by the Drosophila PGC-1 homolog. Cell Metab. 14, 623–634 (2011).
Jager, S., Handschin, C., St-Pierre, J. & Spiegelman, B. M. AMP-activated protein kinase (AMPK) action in skeletal muscle via direct phosphorylation of PGC-1α. Proc. Natl Acad. Sci. USA 104, 12017–12022 (2007).
Narkar, V. A. et al. AMPK and PPARδ agonists are exercise mimetics. Cell 134, 405–415 (2008).
Nakashima, K. & Yakabe, Y. AMPK activation stimulates myofibrillar protein degradation and expression of atrophy-related ubiquitin ligases by increasing FOXO transcription factors in C2C12 myotubes. Biosci. Biotechnol. Biochem. 71, 1650–1656 (2007).
Krawiec, B. J., Nystrom, G. J., Frost, R. A., Jefferson, L. S. & Lang, C. H. AMP-activated protein kinase agonists increase mRNA content of the muscle-specific ubiquitin ligases MAFbx and MuRF1 in C2C12 cells. Am. J. Physiol. Endocrinol. Metab. 292, E1555–E1567 (2007).
Meder, B. et al. JunB-CBFβ signaling is essential to maintain sarcomeric Z-disc structure and when defective leads to heart failure. J. Cell Sci. 123, 2613–2620 (2010).
Finkel, T., Deng, C. X. & Mostoslavsky, R. Recent progress in the biology and physiology of sirtuins. Nature 460, 587–591 (2009).
Lavu, S., Boss, O., Elliott, P. J. & Lambert, P. D. Sirtuins—novel therapeutic targets to treat age-associated diseases. Nature Rev. Drug Discov. 7, 841–853 (2008).
Haigis, M. C. & Sinclair, D. A. Mammalian sirtuins: biological insights and disease relevance. Annu. Rev. Pathol. 5, 253–295 (2010).
Amat, R. et al. SIRT1 controls the transcription of the peroxisome proliferator-activated receptor-γ Co-activator-1α (PGC-1α) gene in skeletal muscle through the PGC-1α autoregulatory loop and interaction with MyoD. J. Biol. Chem. 284, 21872–21880 (2009).
Rathbone, C. R., Booth, F. W. & Lees, S. J. Sirt1 increases skeletal muscle precursor cell proliferation. Eur. J. Cell Biol. 88, 35–44 (2009).
McPherron, A. C., Lawler, A. M. & Lee, S. J. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature 387, 83–90 (1997). This is the classic paper describing myostatin as a factor limiting muscle size.
Kota, J. et al. Follistatin gene delivery enhances muscle growth and strength in nonhuman primates. Sci. Transl. Med. 1, 6ra15 (2009).
Bogdanovich, S. et al. Functional improvement of dystrophic muscle by myostatin blockade. Nature 420, 418–421 (2002).
Hamrick, M. W. et al. Recombinant myostatin (GDF-8) propeptide enhances the repair and regeneration of both muscle and bone in a model of deep penetrant musculoskeletal injury. J. Trauma 69, 579–583 (2010).
Anderson, S. B., Goldberg, A. L. & Whitman, H. Identification of a novel pool of etracellular pro-myostatin in skeletal muscle. J. Biol. Chem. 283, 7027–7035 (2008).
Zhang, L. et al. Pharmacological inhibition of myostatin suppresses systemic inflammation and muscle atrophy in mice with chronic kidney disease. FASEB J. 25, 1653–1663 (2011).
Zhang, C. et al. Inhibition of myostatin protects against diet-induced obesity by enhancing fatty acid oxidation and promoting a brown adipose phenotype in mice. Diabetologia 55, 183–193 (2012).
Akpan, I. et al. The effects of a soluble activin type IIB receptor on obesity and insulin sensitivity. Int. J. Obes. (Lond.) 33, 1265–1273 (2009).
Wang, Q. & McPherron, A. C. Myostatin inhibition induces muscle fibre hypertrophy prior to satellite cell activation. J. Physiol. 590, 2151–2165 (2012).
Gilson, H. et al. Follistatin induces muscle hypertrophy through satellite cell proliferation and inhibition of both myostatin and activin. Am. J. Physiol. Endocrinol. Metab. 297, E157–E164 (2009).
Dunshea, F. R., Chung, C. S., Owens, P. C., Ballard, J. F. & Walton, P. E. Insulin-like growth factor-I and analogues increase growth in artificially-reared neonatal pigs. Br. J. Nutr. 87, 587–593 (2002).
Creaney, L. & Hamilton, B. Growth factor delivery methods in the management of sports injuries: the state of play. Br. J. Sports Med. 42, 314–320 (2008).
Garcia, J. M. & Polvino, W. J. Pharmacodynamic hormonal effects of anamorelin, a novel oral ghrelin mimetic and growth hormone secretagogue in healthy volunteers. Growth Horm. IGF Res. 19, 267–273 (2009).
Porporato, P. E. et al. Acylated and unacylated ghrelin impair skeletal muscle atrophy in mice. J. Clin. Invest. 123, 611–622 (2013).
Vestergaard, E. T., Moller, N. & Jorgensen, J. O. Acute peripheral tissue effects of ghrelin on interstitial levels of glucose, glycerol, and lactate: a microdialysis study in healthy human subjects. Am. J. Physiol. Endocrinol. Metab. 304, E1273–E1280 (2013).
Lynch, G. S. & Ryall, J. G. Role of β-adrenoceptor signaling in skeletal muscle: implications for muscle wasting and disease. Physiol. Rev. 88, 729–767 (2008).
Hinkle, R. T. et al. Skeletal muscle hypertrophy and anti-atrophy effects of clenbuterol are mediated by the β2-adrenergic receptor. Muscle Nerve 25, 729–734 (2002).
Kline, W. O., Panaro, F. J., Yang, H. & Bodine, S. C. Rapamycin inhibits the growth and muscle-sparing effects of clenbuterol. J. Appl. Physiol. 102, 740–747 (2007) (1985).
Busquets, S. et al. Anticachectic effects of formoterol: a drug for potential treatment of muscle wasting. Cancer Res. 64, 6725–6731 (2004).
Ryall, J. G., Schertzer, J. D. & Lynch, G. S. Attenuation of age-related muscle wasting and weakness in rats after formoterol treatment: therapeutic implications for sarcopenia. J. Gerontol. A Biol. Sci. Med. Sci. 62, 813–823 (2007).
Berdeaux, R. & Stewart, R. cAMP signaling in skeletal muscle adaptation: hypertrophy, metabolism, and regeneration. Am. J. Physiol. Endocrinol. Metab. 303, E1–E17 (2012).
Goncalves, D. A. et al. Clenbuterol suppresses proteasomal and lysosomal proteolysis and atrophy-related genes in denervated rat soleus muscles independently of Akt. Am. J. Physiol. Endocrinol. Metab. 302, E123–E133 (2012).
Brett, J., Dawson, A. H. & Brown, J. A. Clenbuterol toxicity: a NSW poisons information centre experience. Med. J. Aust. 200, 219–221 (2014).
Rabe, K. F. Update on roflumilast, a phosphodiesterase 4 inhibitor for the treatment of chronic obstructive pulmonary disease. Br. J. Pharmacol. 163, 53–67 (2011).
Hatzelmann, A. et al. The preclinical pharmacology of roflumilast—a selective, oral phosphodiesterase 4 inhibitor in development for chronic obstructive pulmonary disease. Pulm Pharmacol. Ther. 23, 235–256 (2010).
Endres, S. et al. Cyclic nucleotides differentially regulate the synthesis of tumour necrosis factor-α and interleukin-1 β by human mononuclear cells. Immunology 72, 56–60 (1991).
Beghe, B., Rabe, K. F. & Fabbri, L. M. Phosphodiesterase-4 inhibitor therapy for lung diseases. Am. J. Respir. Crit. Care Med. 188, 271–278 (2013). References 225–228 describe the clinical use of PDE4 inhibitors for the treatment of COPD.
Combaret, L. et al. Torbafylline (HWA 448) inhibits enhanced skeletal muscle ubiquitin-proteasome-dependent proteolysis in cancer and septic rats. Biochem. J. 361, 185–192 (2002).
Vary, T. et al. Pentoxifylline improves insulin action limiting skeletal muscle catabolism after infection. J. Endocrinol. 163, 15–24 (1999).
Baviera, A. M., Zanon, N. M., Carvalho Navegantes, L. C., Migliorini, R. H. & do Carmo Kettelhut, I. Pentoxifylline inhibits Ca2+-dependent and ATP proteasome-dependent proteolysis in skeletal muscle from acutely diabetic rats. Am. J. Physiol. Endocrinol. Metab. 292, E702–E708 (2007).
Hinkle, R. T., Dolan, E., Cody, D. B., Bauer, M. B. & Isfort, R. J. Phosphodiesterase 4 inhibition reduces skeletal muscle atrophy. Muscle Nerve 32, 775–781 (2005).
Barnette, M. S. & Underwood, D. C. New phosphodiesterase inhibitors as therapeutics for the treatment of chronic lung disease. Curr. Opin. Pulm. Med. 6, 164–169 (2000).
Spina, D. Phosphodiesterase-4 inhibitors in the treatment of inflammatory lung disease. Drugs 63, 2575–2594 (2003).
Goncalves, D. A. et al. Mechanisms involved in 3′,5′-cyclic adenosine monophosphate-mediated inhibition of the ubiquitin-proteasome system in skeletal muscle. Endocrinology 150, 5395–5404 (2009).
Lira, E. C. et al. Cyclic adenosine monophosphate-phosphodiesterase inhibitors reduce skeletal muscle protein catabolism in septic rats. Shock 27, 687–694 (2007).
Lira, E. C. et al. Phosphodiesterase-4 inhibition reduces proteolysis and atrogenes expression in rat skeletal muscles. Muscle Nerve 44, 371–381 (2011).
Sinha-Hikim, I. et al. Testosterone-induced increase in muscle size in healthy young men is associated with muscle fiber hypertrophy. Am. J. Physiol. Endocrinol. Metab. 283, E154–E164 (2002).
Griggs, R. C. et al. Effect of testosterone on muscle mass and muscle protein synthesis. J. Appl. Physiol. 66, 498–503 (1989).
Sinha-Hikim, I., Roth, S. M., Lee, M. I. & Bhasin, S. Testosterone-induced muscle hypertrophy is associated with an increase in satellite cell number in healthy, young men. Am. J. Physiol. Endocrinol. Metab. 285, E197–E205 (2003).
Curran, M. J. & Bihrle, W. 3rd. Dramatic rise in prostate-specific antigen after androgen replacement in a hypogonadal man with occult adenocarcinoma of the prostate. Urology 53, 423–424 (1999).
Bhasin, S. et al. Drug insight: Testosterone and selective androgen receptor modulators as anabolic therapies for chronic illness and aging. Nature Clin. Pract. Endocrinol. Metab. 2, 146–159 (2006).
Narayanan, R., Mohler, M. L., Bohl, C. E., Miller, D. D. & Dalton, J. T. Selective androgen receptor modulators in preclinical and clinical development. Nucl. Recept. Signal 6, e010 (2008).
Zilbermint, M. F. & Dobs, A. S. Nonsteroidal selective androgen receptor modulator Ostarine in cancer cachexia. Future Oncol. 5, 1211–1220 (2009). References 242–244 describe the use of SARMs in pharmacological therapy of wasting in several diseases.
Murphy, K. T., Cobani, V., Ryall, J. G., Ibebunjo, C. & Lynch, G. S. Acute antibody-directed myostatin inhibition attenuates disuse muscle atrophy and weakness in mice. J. Appl. Physiol. 110, 1065–1072 (2011).
Miki, K. et al. Ghrelin treatment of cachectic patients with chronic obstructive pulmonary disease: a multicenter, randomized, double-blind, placebo-controlled trial. PLoS ONE 7, e35708 (2012).
Garcia, J. M., Friend, J. & Allen, S. Therapeutic potential of anamorelin, a novel, oral ghrelin mimetic, in patients with cancer-related cachexia: a multicenter, randomized, double-blind, crossover, pilot study. Support Care Cancer 21, 129–137 (2013).
Dalton, J. T. et al. The selective androgen receptor modulator GTx-024 (enobosarm) improves lean body mass and physical function in healthy elderly men and postmenopausal women: results of a double-blind, placebo-controlled phase II trial. J. Cachexia Sarcopenia Muscle 2, 153–161 (2011).
Dobs, A. S. et al. Effects of enobosarm on muscle wasting and physical function in patients with cancer: a double-blind, randomised controlled phase 2 trial. Lancet Oncol. 14, 335–345 (2013).
Stewart Coats, A. J. et al. The ACT-ONE trial, a multicentre, randomised, double-blind, placebo-controlled, dose-finding study of the anabolic/catabolic transforming agent, MT-102 in subjects with cachexia related to stage III and IV non-small cell lung cancer and colorectal cancer: study design. J. Cachexia Sarcopenia Muscle 2, 201–207 (2011).
Basaria, S. et al. The safety, pharmacokinetics, and effects of LGD-4033, a novel nonsteroidal oral, selective androgen receptor modulator, in healthy young men. J. Gerontol. A Biol. Sci. Med. Sci. 68, 87–95 (2013).
Zhang, L. et al. Stat3 activation links a C/EBPδ to myostatin pathway to stimulate loss of muscle mass. Cell Metab. 18, 368–379 (2013).
Lee, S.J. et al. Regulation of muscle growth by multiple ligands signaling through activin type II receptors. Proc. Natl. Acad. Sci. USA. 13, 18117–18122 (2005).
Acknowledgements
The research of A.L.G. is supported in part by grants from the US National Institutes of Health (ARO55255) and the Muscular Dystrophy Association. J.A.N. is supported by a Wellcome Trust Senior Clinical Research Fellowship and S.C. receives a stipend from the International Sephardic Education Foundation (ISEF).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Cachexia
-
Severe loss of body weight (especially muscle mass), with or without the loss of fat. Cachexia is associated with serious disease, in particular cancer.
- Myofibrillar
-
Consisting of myofibrils. Myofibrils are the organizational units in skeletal muscle composed of aligned filaments that enable contraction. Myofibrils contain mainly myosin in the thick filaments and actin in the thin filaments plus many less abundant regulatory proteins.
- Atrogenes
-
Atrophy-related genes that are similarly induced or suppressed in all types of atrophy in skeletal muscles.
- Catabolic diseases
-
Diseases associated with marked weight loss, particularly loss of muscle mass and strength owing to the accelerated destruction of muscle proteins.
- Sarcomere
-
The repeated structural and contractile unit along the length of a myofibril delimited by the Z-bands.
- Z-bands
-
The boundaries of sarcomeres where desmin filaments are aligned and thin (actin) filaments are anchored.
- N-end rule ubiquitylation system
-
A pathway for ubiquitylation that targets degradation proteins with unusual amino-terminal residues, which may be generated by proteolytic cleavage of normal cell proteins.
- Sarcopenia
-
The gradual loss of skeletal muscle mass seen in aged humans and animals.
Rights and permissions
About this article
Cite this article
Cohen, S., Nathan, J. & Goldberg, A. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov 14, 58–74 (2015). https://doi.org/10.1038/nrd4467
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4467
This article is cited by
-
Impact of exercise training and diet therapy on the physical fitness, quality of life, and immune response of people living with HIV/AIDS: a randomized controlled trial
BMC Public Health (2024)
-
Transcriptional programming of translation by BCL6 controls skeletal muscle proteostasis
Nature Metabolism (2024)
-
A protocol for randomized controlled trial on multidisciplinary interventions for mobility limitation in the older adults (M-MobiLE)
BMC Geriatrics (2023)
-
Eldecalcitol prevents muscle loss and osteoporosis in disuse muscle atrophy via NF-κB signaling in mice
Skeletal Muscle (2023)
-
Exercise mitigates Dapagliflozin-induced skeletal muscle atrophy in STZ-induced diabetic rats
Diabetology & Metabolic Syndrome (2023)