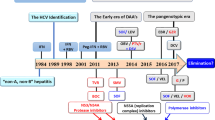
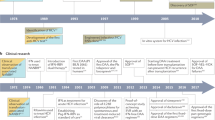
Key Points
-
Hepatitis C is the only chronic viral infection that can be cured through drug therapy.
-
The current standard of care in chronic hepatitis C virus (HCV) infection is pegylated interferon plus ribavirin with the addition of a protease inhibitor in genoytpe 1 infection.
-
Numerous novel direct-acting antiviral (DAA) drugs are in advanced clinical development and are anticipated to reach the market within the next few years.
-
Advanced novel DAAs target various HCV factors: the NS3/4A protease, the NS5A RNA binding protein and the NS5B RNA-dependent RNA polymerase
-
Sustained virological response or cure of HCV infection is possible using all-oral combinations of DAAs.
-
Nucleoside and nucleotide inhibitors of the NS5B polymerase usually have a high barrier to resistance and good activity against different HCV genotypes and thus are likely candidates as backbone drugs in future all-oral regimens.
Abstract
Almost 25 years after the hepatitis C virus (HCV) was identified, and following intense research and development efforts, a large number of direct-acting antiviral drugs are now beginning to reach patient care. Accordingly, the way in which care is delivered is evolving at a breath-taking pace. Here, we review the current and upcoming treatment options for HCV, describe the key challenges facing clinicians and drug developers and discuss how the landscape in the HCV arena will change over the coming years.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Tabor, E. et al. Transmission of non-A, non-B hepatitis from man to chimpanzee. Lancet 1, 463–466 (1978).
Hoofnagle, J. H. et al. Treatment of chronic non-A, non-B hepatitis with recombinant human alpha interferon. A preliminary report. N. Engl. J. Med. 315, 1575–1578 (1986).
Choo, Q. L. et al. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244, 359–362 (1989). Discovery of the HCV genome.
Lamarre, D. et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 426, 186–189 (2003). First in vivo data in humans on an HCV DAA.
Hinrichsen, H. et al. Short-term antiviral efficacy of BILN 2061, a hepatitis C virus serine protease inhibitor, in hepatitis C genotype 1 patients. Gastroenterology 127, 1347–1355 (2004).
Reiser, M. et al. Antiviral efficacy of NS3-serine protease inhibitor BILN-2061 in patients with chronic genotype 2 and 3 hepatitis C. Hepatology 41, 832–835 (2005).
Poordad, F. et al. Boceprevir for untreated chronic HCV genotype 1 infection. N. Engl. J. Med. 364, 1195–1206 (2011).
Bacon, B. R. et al. Boceprevir for previously treated chronic HCV genotype 1 infection. N. Engl. J. Med. 364, 1207–1217 (2011).
Jacobson, I. M. et al. Telaprevir for previously untreated chronic hepatitis C virus infection. N. Engl. J. Med. 364, 2405–2416 (2011).
Zeuzem, S. et al. Telaprevir for retreatment of HCV infection. N. Engl. J. Med. 364, 2417–2428 (2011).
Zou, S. et al. Prevalence, incidence, and residual risk of human immunodeficiency virus and hepatitis C virus infections among United States blood donors since the introduction of nucleic acid testing. Transfusion 50, 1495–1504 (2010).
Klevens, R. M., Hu, D. J., Jiles, R. & Holmberg, S. D. Evolving epidemiology of hepatitis C virus in the United States. Clin. Infect. Dis. 55 (Suppl. 1), S3–S9 (2012).
Kolykhalov, A. A. et al. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277, 570–574 (1997).
Blight, K. J., Kolykhalov, A. A., Reed, K. E., Agapov, E. V. & Rice, C. M. Molecular virology of hepatitis C virus: an update with respect to potential antiviral targets. Antivir. Ther. 3, 71–81 (1998).
Lohmann, V. et al. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285, 110–113 (1999). First description of the HCV replicon system.
Lindenbach, B. D. et al. Cell culture-grown hepatitis C virus is infectious in vivo and can be recultured in vitro. Proc. Natl Acad. Sci. USA 103, 3805–3809 (2006).
Maylin, S. et al. Eradication of hepatitis C virus in patients successfully treated for chronic hepatitis C. Gastroenterology 135, 821–829 (2008).
Swain, M. G. et al. A sustained virologic response is durable in patients with chronic hepatitis C treated with peginterferon alfa-2a and ribavirin. Gastroenterology 139, 1593–1601 (2010).
Manns, M. P. et al. Long-term clearance of hepatitis C virus following interferon alfa-2b or peginterferon α-2b, alone or in combination with ribavirin. J. Viral Hepat. 3 Mar 2013 (doi:10.1111/jvh.12074).
Pilot-Matias, T. et al. Characterization of resistant variants in NS3 and NS5B detected in subjects treated with ABT-450/r, ribavirin, and either ABT-072 or ABT-333 in the pilot and co-pilot studies who experienced virologic breakthrough or relapse. Hepatology 56, 569A–570A (2012).
van der Meer, A. J. et al. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308, 2584–2593 (2012). First demonstration that SVR reduces overall mortality.
Poynard, T. et al. Meta-analysis of interferon randomized trials in the treatment of viral hepatitis C: effects of dose and duration. Hepatology 24, 778–789 (1996).
Manns, M. P. et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet 358, 958–965 (2001). First Phase III study to describe PEG-IFNα in combination with ribavirin as a new standard of care from 2001 to 2011.
Fried, M. W. et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N. Engl. J. Med. 347, 975–982 (2002).
Wakita, T. et al. Production of infectious hepatitis C virus in tissue culture from a cloned viral genome. Nature Med. 11, 791–796 (2005).
Lindenbach, B. D. et al. Complete replication of hepatitis C virus in cell culture. Science 309, 623–626 (2005).
Zhong, J. et al. Robust hepatitis C virus infection in vitro. Proc. Natl Acad. Sci. USA 102, 9294–9299 (2005).
Love, R. A. et al. The crystal structure of hepatitis C virus NS3 proteinase reveals a trypsin-like fold and a structural zinc binding site. Cell 87, 331–342 (1996).
Kim, J. L. et al. Crystal structure of the hepatitis C virus NS3 protease domain complexed with a synthetic NS4A cofactor peptide. Cell 87, 343–355 (1996).
Tellinghuisen, T. L., Marcotrigiano, J. & Rice, C. M. Structure of the zinc-binding domain of an essential component of the hepatitis C virus replicase. Nature 435, 374–379 (2005).
Lesburg, C. A. et al. Crystal structure of the RNA-dependent RNA polymerase from hepatitis C virus reveals a fully encircled active site. Nature Struct. Biol. 6, 937–943 (1999).
Bressanelli, S. et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Proc. Natl Acad. Sci. USA 96, 13034–13039 (1999).
Ago, H. et al. Crystal structure of the RNA-dependent RNA polymerase of hepatitis C virus. Structure 7, 1417–1426 (1999).
Lindenbach, B., Thiel, H. J. & Rice, C. M. in Fields Virology 5th edn (Lippincott-Raven, 2007).
Alvisi, G., Madan, V. & Bartenschlager, R. Hepatitis C virus and host cell lipids: an intimate connection. RNA Biol. 8, 258–269 (2011).
Ploss, A. & Evans, M. J. Hepatitis C virus host cell entry. Curr. Opin. Virol. 2, 14–19 (2012).
Romero-Brey, I. et al. Three-dimensional architecture and biogenesis of membrane structures associated with hepatitis C virus replication. PLoS Pathog. 8, e1003056 (2012).
O'Brien, T. R. Interferon-alfa, interferon-lambda and hepatitis C. Nature Genet. 41, 1048–1050 (2009).
Muir, A. J. et al. Peginterferon lambda-1a (Lambda) compared to Peginterferon alfa-2a (Alfa) in treatment-naive patients with HCV genotypes (GT) 1 or 4: SVR24. Results From EMERGE Phase 2b. Hepatology 56 (Suppl.), 299A (2012).
Zeuzem, S. et al. Peginterferon lambda-1a (Lambda) compared to Peginterferon alfa-2a (Alfa) in treatment-naive patients with HCV genotypes (G) 2 or 3: first SVR24 results from EMERGE Phase IIB. J. Hepatol. 56 (Suppl. 2), S5–S6 (2012).
Moresco, E. M., LaVine, D. & Beutler, B. Toll-like receptors. Curr. Biol. 21, R488–R493 (2011).
Lorenz, I. C., Marcotrigiano, J., Dentzer, T. G. & Rice, C. M. Structure of the catalytic domain of the hepatitis C virus NS2-3 protease. Nature 442, 831–835 (2006).
Sarrazin, C. & Zeuzem, S. Resistance to direct antiviral agents in patients with hepatitis C virus infection. Gastroenterology 138, 447–462 (2010).
McCown, M. F., Rajyaguru, S., Kular, S., Cammack, N. & Najera, I. GT-1a or GT-1b subtype-specific resistance profiles for hepatitis C virus inhibitors telaprevir and HCV-796. Antimicrob. Agents Chemother. 53, 2129–2132 (2009).
Ciesek, S., von Hahn, T. & Manns, M. P. Second-wave protease inhibitors: choosing an heir. Clin. Liver Dis. 15, 597–609 (2011).
Manns, M. et al. Simeprevir (TMC435) with peginterferon/ribavirin for treatment of chronic HCV genotype-1 infection in treatment-naive patients: results from QUEST-2, a Phase III trial. J. Hepatol. 58 (Suppl. 1), S568 (2013).
Jacobson, I. et al. Simeprevir (TMC435) with peginterferon/ribavirin for chronic HCV genotype-1 infection in treatment-naive patients: results from QUEST-1, a Phase III trial. J. Hepatol. 58 (Suppl. 1), S574 (2013).
Sulkowski, M. S. et al. Faldaprevir combined with peginterferon alfa-2a and ribavirin in treatment-naive patients with chronic genotype-1 HCV: SILEN-C1 trial. Hepatology 28 Jan 2013 (doi: 10.1002/hep.26276).
Sulkowski, M. S. et al. Faldaprevir combined with peginterferon alfa-2a and ribavirin in chronic HCV genotype-1 patients with prior nonresponse: SILEN-C2 trial. Hepatology 15 Mar 2013 (doi:10.1002/hep.26386).
Manns, M. et al. High sustained viral response at 12- and 24-week follow-up of MK-5172 with pegylated interferon alfa-2b and ribavirin (PR) in HCV genotype 1 treatment-naive non-cirrhotic patients. J. Hepatol. 58 (Suppl. 1), S30 (2013).
Pawlotsky, J. M., Najera, I. & Jacobson, I. Resistance to mericitabine, a nucleoside analogue inhibitor of HCV RNA-dependent RNA polymerase. Antivir. Ther. 17, 411–423 (2012).
Bristol-Myers Squibb. Press release 23 Aug 2012: Bristol-Myers Squibb discontinues development of BMS-986094, an investigational NS5B nucleotide for the treatment of hepatitis C. BMS website[online], (2012).
Lawitz, E. et al. Sofosbuvir for previously untreated chronic hepatitis C infection. N. Engl. J. Med. 368, 1878–1887 (2013). Phase II data on sofosbuvir in all genotypes from the NEUTRINO and FISSION studies.
Everson, G. T. et al. The NS5A inhibitor GS-5885 is safe and well-tolerated in over 1000 patients treated in Phase 2 studies. Hepatology 56 (Suppl.), 572A–573A (2012).
Sulkowski, M. S. et al. High rate of sustained virologic response with the all-oral combination of daclatasvir (NS5A inhibitor) plus sofosbuvir (nucleotide NS5B inhibitor), with or without ribavirin, in treatment-naive patients chronically Infected with HCV genotype 1, 2, or 3. Hepatology, 56 (Suppl.), 1516A–1517A (2012).
Poordad, F. et al. ABT-072 or ABT-333 combined with pegylated interferon/ribavirin after 3-day monotherapy in HCV genotype 1 (GT1)-infected treatment-naive subjects: 12-week sustained virologic response (SVR12) and safety results. J. Hepatol. 56 (Suppl. 2), S478 (2012).
Guedj, J. et al. Modeling shows that the NS5A inhibitor daclatasvir has two modes of action and yields a shorter estimate of the hepatitis C virus half-life. Proc. Natl Acad. Sci. USA 110, 3991–3996 (2013).
Gao, M. et al. Chemical genetics strategy identifies an HCV NS5A inhibitor with a potent clinical effect. Nature 465, 96–100 (2010).
Hézode, C. et al. Daclatasvir, an NS5A replication complex inhibitor, combined With Peginterferon alfa-2a & ribavirin in treatment-naive, HCV-genotype 1 or 4 subjects: Phase 2b COMMAND-1 SVR12 results. Hepatology 56 (Suppl.), 553A–554A (2012).
Fridell, R. A. et al. Genotypic and phenotypic analysis of variants resistant to hepatitis C virus nonstructural protein 5A replication complex inhibitor BMS-790052 in humans: in vitro and in vivo correlations. Hepatology 54, 1924–1935 (2011).
Lok, A. S. et al. Sustained virologic response in chronic HCV genotype (GT) 1-Infected null responders with combination of daclatasvir (DCV; NS5A inhibitor) and asunaprevir (ASV; NS3 Inhibitor) with or without Peginterferon alfa-2a/ribavirin (PEG/RBV). Hepatology 56 (Suppl.), 230A–231A (2012).
Thompson, A. et al. GS-5885 + GS-9451 + peginterferon and ribavirin (PR) for six or twelve weeks achieves high SVR12 rates in treatment-naive genotype 1 IL28B CC patients. J. Hepatol. 58 (Suppl. 1), S29 (2013).
Einav, S. et al. Discovery of a hepatitis C target and its pharmacological inhibitors by microfluidic affinity analysis. Nature Biotech. 26, 1019–1027 (2008).
von Hahn, T., Ciesek, S. & Manns, M. P. Arrest all accessories — inhibition of hepatitis C virus by compounds that target host factors. Discov. Med. 12, 237–244 (2011).
Paeshuyse, J. et al. The non-immunosuppressive cyclosporin DEBIO-025 is a potent inhibitor of hepatitis C virus replication in vitro. Hepatology 43, 761–770 (2006).
Ciesek, S. et al. The green tea polyphenol, epigallocatechin-3-gallate, inhibits hepatitis C virus entry. Hepatology 54, 1947–1955 (2011).
Nag, A., Robotham, J. M. & Tang, H. Suppression of viral RNA binding and the assembly of infectious hepatitis C virus particles in vitro by cyclophilin inhibitors. J. Virol. 86, 12616–12624 (2012).
Flisiak, R. et al. The cyclophilin inhibitor Debio 025 combined with PEG IFNα2a significantly reduces viral load in treatment-naive hepatitis C patients. Hepatology 49, 1460–1468 (2009).
Pawlotsky, J.-M. et al. Alisporivir plus ribavirin achieves high rates of sustained HCV clearance (SVR24) as interferon (IFN)-free or IFN-add-on regimen in treatment-naive patients with HCV GT2 or GT3: final results from VITAL-1 study. Hepatology 56 (Suppl.), 309A–310A (2012).
Patel, H. & Heathcote, E. J. Sustained virological response with 29 days of Debio 025 monotherapy in hepatitis C virus genotype 3. Gut 60, 879 (2011).
Lanford, R. E. et al. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science 327, 198–201 (2010).
Lindow, M. & Kauppinen, S. Discovering the first microRNA-targeted drug. J. Cell Biol. 199, 407–412 (2012).
Janssen, H. L. et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 368, 1685–1694 (2013). First study to describe efficacy of an miRNA-directed treatment in humans.
Syder, A. J. et al. Small molecule scavenger receptor BI antagonists are potent HCV entry inhibitors. J. Hepatol. 54, 48–55 (2011).
Lok, A. S. et al. Preliminary study of two antiviral agents for hepatitis C genotype 1. N. Engl. J. Med. 366, 216–224 (2012). Proof of concept that SVR can be achieved with an all-oral DAA combination.
Chayama, K. et al. Dual therapy with the nonstructural protein 5A inhibitor, daclatasvir, and the nonstructural protein 3 protease inhibitor, asunaprevir, in hepatitis C virus genotype 1b-infected null responders. Hepatology 55, 742–748 (2012).
Poordad, F. et al. Exploratory study of oral combination antiviral therapy for hepatitis C. N. Engl. J. Med. 368, 45–53 (2013).
Zeuzem, S. et al. The protease inhibitor, GS-9256, and non-nucleoside polymerase inhibitor tegobuvir alone, with ribavirin, or pegylated interferon plus ribavirin in hepatitis C. Hepatology 55, 749–758 (2012).
Kowdley, K. V. et al. A 12-week interferon-free treatment regimen with ABT-450/r, ABT-267, ABT-333 and ribavirin achieves SVR rates (observed data) of 99% in treatment-naive patients and 93% in prior null responders with HCV genotype 1 infection. Hepatology 56 (Suppl.), 1515A–1516A (2012).
Kowdley, K. V. et al. Safety & efficacy of interferon-free regimens of ABT-450/r, ABT-267, ABT-333 +/− ribavirin in patients with chronic HCV GT1 infection: results from the AVIATOR study. J. Hepatol. 58 (Suppl. 1), S2 (2013).
Sulkowski, M. S. et al. Sustained virologic response with daclatasvir plus sofosbuvir ± ribavirin (RBV) in chronic HCV genotype (GT) 1-infected patients who previously failed telaprevir (TVR) or boceprevir (BOC). J. Hepatol. 58 (Suppl. 1), S570 (2013).
Fontana, R. J. et al. Sofosbuvir and daclatasvir combination therapy in a liver transplant recipient with severe recurrent cholestatic hepatitis C. Am. J. Transplant. 17 Apr 2013 (doi:10.1111/ajt.12209). Demonstration that an all-oral DAA combination can be used in post-transplantation cholestatic hepatitis.
Lawitz, E. et al. Suppression of viral load through 4 weeks post-treatment results of a once-daily regimen of simeprevir + sofosbuvir with or without ribavirin in hepatitis C virus GT 1 null responders. Conference on Retroviruses and Opportunistic Infections website[online], (2013).
Gane, E. et al. Phase 3 randomized controlled trial of all-oral treatment with sofosbuvir + ribavirin for 12 weeks compared to 24 weeks of peg + ribavirin in treatment-naive GT 2/3 HCV-infected patients (FISSION). J. Hepatol. 58 (Suppl. 1), S3 (2013).
Jacobson, I. M. et al. Sofosbuvir for hepatitis C genotype 2 or 3 in patients without treatment options. N. Engl. J. Med. 368, 1867–1877 (2013). Phase III data on sofosbuvir in genotypes 2/3.
Zeuzem, S. et al. SOUND-C2: SVR4, 12 & 24 concordance in genotype (GT) 1 HCV. patients receiving interferon (IFN)-free treatment with the HCV NS3/4A protease inhibitor BI 201335 & the NS5B polymerase inhibitor B I 207127. Hepatology 56 (Suppl.), 569A (2012).
Soriano, V. et al. Efficacy and safety of the interferon (IFN)-free combination of BI 201335 + BI 207127 ±ribavirin (RBV) in treatment-naive patients with HCV genotype (GT) 1 infection and compensated liver cirrhosis: Results from the SOUND-C2 study. Hepatology 56 (Suppl.), 234A (2012).
Everson, G. T. et al. An interferon-free, ribavirin-free 12-week regimen of daclatasvir (DCV), asunaprevir (ASV), and BMS-791325 yielded SVR4 of 94% in treatment-naive patients with genotype (GT) 1 chronic hepatitis C virus (HCV) infection. Hepatology 56 (Suppl.), 1517A–1518A (2012).
Pearlman, B. et al. Hepatitis C virus (HCV) genotype 1 (G1) infection with low viral load (LVL) and rapid virologic response (RVR) to peginterferon and ribavirin (PEG/RBV) can be treated without a protease inhibitor (PI), irrespective of IL-28B status or patient ethnicity. Hepatology 56 (Suppl.), 268A (2012).
Camma, C. et al. Cost-effectiveness of boceprevir or telaprevir for untreated patients with genotype 1 chronic hepatitis C. Hepatology 56, 850–860 (2012).
Volk, M. L., Tocco, R., Saini, S. & Lok, A. S. Public health impact of antiviral therapy for hepatitis C in the United States. Hepatology 50, 1750–1755 (2009).
Crespo, G., Marino, Z., Navasa, M. & Forns, X. Viral hepatitis in liver transplantation. Gastroenterology 142, 1373–1383.e1 (2012).
Simmonds, P. et al. Consensus proposals for a unified system of nomenclature of hepatitis C virus genotypes. Hepatology 42, 962–973 (2005).
Simmonds, P. Genetic diversity and evolution of hepatitis C virus — 15 years on. J. Gen. Virol. 85, 3173–3188 (2004).
Farci, P. New insights into the HCV quasispecies and compartmentalization. Semin. Liver Dis. 31, 356–374 (2011).
Probst, A. et al. Role of hepatitis C virus genotype 3 in liver fibrosis progression — a systematic review and meta-analysis. J. Viral Hepat. 18, 745–759 (2011).
Sarin, S. K. & Kumar, C. K. Treatment of patients with genotype 3 chronic hepatitis C — current and future therapies. Liver Int. 32 (Suppl. 1), 141–145 (2012).
Prokunina-Olsson, L. et al. A variant upstream of IFNL3 (IL28B) creating a new interferon gene IFNL4 is associated with impaired clearance of hepatitis C virus. Nature Genet. 45, 164–171 (2013). Describes a pkossible mechanism behind the IL28B variant.
Ge, D. et al. Genetic variation in IL28B predicts hepatitis C treatment-induced viral clearance. Nature 461, 399–401 (2009).
Khakoo, S. I. et al. HLA and NK cell inhibitory receptor genes in resolving hepatitis C virus infection. Science 305, 872–874 (2004).
Fellay, J. et al. ITPA gene variants protect against anaemia in patients treated for chronic hepatitis C. Nature 464, 405–408 (2010).
Ochi, H. et al. ITPA polymorphism affects ribavirin-induced anemia and outcomes of therapy — a genome-wide study of Japanese HCV virus patients. Gastroenterology 139, 1190–1197 (2010).
Valenti, L. et al. Patatin-like phospholipase domain-containing 3 I148M polymorphism, steatosis, and liver damage in chronic hepatitis C. Hepatology 53, 791–799 (2011).
Falleti, E. et al. Vitamin D binding protein gene polymorphisms and baseline vitamin D levels as predictors of antiviral response in chronic hepatitis C. Hepatology 56, 1641–1650 (2012).
Lange, C. M. et al. Vitamin D deficiency and a CYP27B1-1260 promoter polymorphism are associated with chronic hepatitis C and poor response to interferon-alfa based therapy. J. Hepatol. 54, 887–893 (2011).
Westhaus, S. et al. Characterization of the inhibition of hepatitis C virus entry by in vitro-generated and patient-derived oxidized low-density lipoprotein. Hepatology 57, 1716–1724 (2012).
Welsch, C., Jesudian, A., Zeuzem, S. & Jacobson, I. New direct-acting antiviral agents for the treatment of hepatitis C virus infection and perspectives. Gut 61 (Suppl. 1), i36–i46 (2012).
Hézode, C. et al. Safety of telaprevir or boceprevir in combination with peginterferon alfa/ribavirin, in cirrhotic non responders. First results of the French early access program (Anrs C020-Cupic). J. Hepatol. 56 (Suppl. 2), S4 (2012).
Rutter, K. et al. Safety of triple therapy with telaprevir or boceprevir in hepatitis C patients with advanced liver disease — predictive factors for sepsis. J. Hepatol. 58 (Suppl. 1), S30 (2013).
Mauss, S. et al. Safety & week 4/12 HCV RNA results of triple combination with telaprevir (TVR)/peginterferon alfa-2a (P)/ribavirin (R), in F3/F4 patients in real-life setting. Hepatology 56 (Suppl. 1), 1037A (2013).
Bichoupan, K. Real world effectiveness of telaprevir-based triple therapy: lower on-treatment virological responses than in RCTs. Hepatology 56 (Suppl. 1), 1044A (2013).
Vierling, J. M. et al. Safety and efficacy of boceprevir/peginterferon/ ribavirin (BOC/P/R) combination therapy for chronic HCV g1 patients with compensated cirrhosis: a meta-analysis of five Phase 3 clinical trials. J. Hepatol. 58 (Suppl. 1), S576–S577 (2013).
Fontaine, H. et al. SVR12 rates and safety of triple therapy including telaprevir or boceprevir in 221 cirrhotic non responders treated in the French early access program (ANRS CO20-CUPIC). J. Hepatol. 58 (Suppl. 1), S27 (2013).
Verna, E. C. et al. High early response rates with protease inhibitor triple therapy in a multicenter cohort of HCV-infected patients awaiting liver transplantation. Hepatology 56 (Suppl. 1), 218A (2013).
Sarrazin, C. et al. Dynamic hepatitis C virus genotypic and phenotypic changes in patients treated with the protease inhibitor telaprevir. Gastroenterology 132, 1767–1777 (2007).
Susser, S. et al. Characterization of resistance to the protease inhibitor boceprevir in hepatitis C virus-infected patients. Hepatology 50, 1709–1718 (2009).
Buti, M. et al. Efficacy of telaprevir dosed twice daily versus every 8 hours by IL28B genotype: results from the Phase III OPTIMIZE study. J. Hepatol. 58 (Suppl. 1), S326 (2013).
Nelson, D. R. et al. High SVR rates (SVR4) for 12-week total telaprevir combination therapy in IL28B CC treatment-naives and prior relapsers with g1 chronic hepatitis C: concise interim analysis. J. Hepatol. 58 (Suppl. 1), S362 (2013).
Hopkins, D. R. Disease eradication. N. Engl. J. Med. 368, 54–63 (2013).
Razavi, H. et al. Chronic hepatitis C virus (HCV) disease burden and cost in the United States. Hepatology 22 Dec 2012 (doi:10.1002/hep.26218).
Alter, H. J. et al. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N. Engl. J. Med. 321, 1494–1500 (1989).
Kuo, G. et al. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244, 362–364 (1989).
Poynard, T. et al. Randomised trial of interferon α2b plus ribavirin for 48 weeks or for 24 weeks versus interferon α2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT). Lancet 352, 1426–1432 (1998).
Davis, G. L. et al. Treatment of chronic hepatitis C with recombinant interferon alfa. A multicenter randomized, controlled trial. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 321, 1501–1506 (1989).
Poynard, T. et al. A comparison of three interferon alfa-2b regimens for the long-term treatment of chronic non-A, non-B hepatitis. Multicenter Study Group. N. Engl. J. Med. 332, 1457–1462 (1995).
McHutchison, J. G. et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N. Engl. J. Med. 339, 1485–1492 (1998).
Zeuzem, S. et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N. Engl. J. Med. 343, 1666–1672 (2000).
Lindsay, K. L. et al. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology 34, 395–403 (2001).
Pockros, P. J. et al. Efficacy and safety of two-dose regimens of peginterferon α-2a compared with interferon α-2a in chronic hepatitis C: a multicenter, randomized controlled trial. Am. J. Gastroenterol. 99, 1298–1305 (2004).
Suppiah, V. et al. IL28B is associated with response to chronic hepatitis C interferon-alpha and ribavirin therapy. Nature Genet. 41, 1100–1104 (2009).
Tanaka, Y. et al. Genome-wide association of IL28B with response to pegylated interferon-α and ribavirin therapy for chronic hepatitis C. Nature Genet. 41, 1105–1109 (2009).
Suzuki, Y. et al. Dual oral therapy with daclatasvir and asunaprevir for patients with HCV genotype 1b infection and limited treatment options. J. Hepatol. 58, 655–662 (2013).
Acknowledgements
The authors are supported by the German Center for Infection Research (DZIF) and Hep-Net, the German network of competence on viral hepatitis, which is a project of the German Liver Foundation. The authors thank S. Ciesek for critical reading of the manuscript and S. Hardtke for editorial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
M.P.M has received fees for consulting work and/or for giving lectures from Roche, Bristol-Myers Squibb, Gilead, Boehringer Ingelheim, Novartis, Merck, Janssen, GlaxoSmithKline, Idenix and Achillion. He has also received research grants from Roche, Gilead, Novartis, Boehringer Ingelheim, Bristol-Myers Squibb, Merck and Janssen. T.v.H. has received speaker's fees from Bristol-Myers Squibb, Merck Sharp & Dohme, and research funding from Novartis and Roche.
Rights and permissions
About this article
Cite this article
Manns, M., von Hahn, T. Novel therapies for hepatitis C — one pill fits all?. Nat Rev Drug Discov 12, 595–610 (2013). https://doi.org/10.1038/nrd4050
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4050
This article is cited by
-
Simple new clinical score to predict hepatocellular carcinoma after sustained viral response with direct-acting antivirals
Scientific Reports (2023)
-
Computational identification of HCV neutralizing antibodies with a common HCDR3 disulfide bond motif in the antibody repertoires of infected individuals
Nature Communications (2022)
-
Crystal structure of SARS-CoV-2 main protease in complex with protease inhibitor PF-07321332
Protein & Cell (2022)
-
AFP and eGFR are related to early and late recurrence of HCC following antiviral therapy
BMC Cancer (2021)
-
Assessing the impact of a combination of sofosbuvir and daclatasvir treatment for hepatitis C virus infection on heart rate, rhythm and heart rate variability using 24-hour ECG monitoring
The Egyptian Heart Journal (2020)