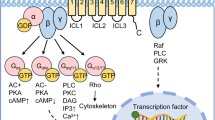
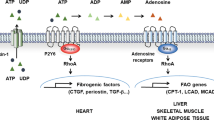
Key Points
-
In recent years, several G protein-coupled receptors (GPCRs) have been identified that are activated by energy substrates such as fatty acids and sucrose, or by metabolic intermediates such as acetate, lactate or ketone bodies.
-
These carbohydrate or lipid metabolites, by activating specific GPCRs, function in a hormone-like fashion in addition to their role as carriers of energy.
-
Metabolite receptors sense metabolic activity or levels of energy substrates and control the secretion of metabolic hormones or regulate the metabolic activity of particular cells.
-
Synthetic ligands of the heterodimeric sweet receptor taste receptor type 1 member 2 (TAS1R2)–TAS1R3, which is a receptor for mono- and disaccharides, are used as artificial sweeteners but may have additional metabolic functions, as TAS1R2–TAS1R3 appears to be also involved in non-gustatory metabolic functions.
-
The long-chain fatty acid receptors free fatty acid receptor 1 (FFA1; (also known as GPR40) and GPR120 (also known as omega-3 fatty acid receptor 1) mediate beneficial effects by promoting glucose-induced insulin secretion and by inhibiting inflammatory signalling in immune cells, respectively.
-
The short-chain fatty acid receptors FFA2 (also known as GPR43) and FFA3 (also known as GPR41) appear to link the gut microbiota to metabolic and immune functions. Whether these receptors can be exploited therapeutically is currently not clear.
-
Succinate receptor 1 (SUCNR1; also known as GPR91), which is activated by the citric acid cycle intermediate succinate (released by cells following cellular stress or hypoxia), is involved in the regulation of blood pressure, retinal angiogenesis and immune functions.
-
Of the hydroxycarboxylic acid receptors, which are activated by ketone bodies or lactate, hydroxycarboxylic acid receptor 2 (HCA2) — which is also activated by nicotinic acid — in particular is of pharmacological interest as it mediates some anti-dyslipidaemic effects as well as anti-inflammatory effects, which can be used to reduce the progression of atherosclerosis and potentially other diseases.
-
Several of these receptors have been tested as targets for drugs to treat particular metabolic diseases, and various synthetic ligands have been developed in recent years. Agonists of FFA1 and HCA2 are currently being tested in clinical trials.
Abstract
Several G protein-coupled receptors (GPCRs) that are activated by intermediates of energy metabolism — such as fatty acids, saccharides, lactate and ketone bodies — have recently been discovered. These receptors are able to sense metabolic activity or levels of energy substrates and use this information to control the secretion of metabolic hormones or to regulate the metabolic activity of particular cells. Moreover, most of these receptors appear to be involved in the pathophysiology of metabolic diseases such as diabetes, dyslipidaemia and obesity. This Review summarizes the functions of these metabolite-sensing GPCRs in physiology and disease, and discusses the emerging pharmacological agents that are being developed to target these GPCRs for the treatment of metabolic disorders.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
References
Rask-Andersen, M., Almen, M. S. & Schioth, H. B. Trends in the exploitation of novel drug targets. Nature Rev. Drug. Discov. 10, 579–590 (2011).
Lefkowitz, R. J. Seven transmembrane receptors: something old, something new. Acta Physiol. (Oxf.) 190, 9–19 (2007).
Oldham, W. M. & Hamm, H. E. How do receptors activate G proteins? Adv. Protein Chem. 74, 67–93 (2007).
Wettschureck, N. & Offermanns, S. Mammalian G proteins and their cell type specific functions. Physiol. Rev. 85, 1159–1204 (2005).
Ahmadian, M., Duncan, R. E. & Sul, H. S. The skinny on fat: lipolysis and fatty acid utilization in adipocytes. Trends Endocrinol. Metab. 20, 424–428 (2009).
Bezaire, V. & Langin, D. Regulation of adipose tissue lipolysis revisited. Proc. Nutr. Soc. 68, 350–360 (2009).
Ahren, B. Islet G protein-coupled receptors as potential targets for treatment of type 2 diabetes. Nature Rev. Drug. Discov. 8, 369–385 (2009). This is an excellent review on the role of GPCRs in the regulation of pancreatic β -cells and their established or potential roles as targets for antidiabetic drugs.
Engelstoft, M. S., Egerod, K. L., Holst, B. & Schwartz, T. W. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell. Metab. 8, 447–449 (2008).
Reimann, F., Tolhurst, G. & Gribble, F. M. G-protein-coupled receptors in intestinal chemosensation. Cell. Metab. 15, 421–431 (2012). This is an excellent review on the role of GPCRs in enteric cells and their function in the regulation of metabolic processes.
Rocha, V. Z. & Libby, P. Obesity, inflammation, and atherosclerosis. Nature Rev. Cardiol. 6, 399–409 (2009).
Lumeng, C. N. & Saltiel, A. R. Inflammatory links between obesity and metabolic disease. J. Clin. Invest. 121, 2111–2117 (2011).
Olefsky, J. M. & Glass, C. K. Macrophages, inflammation, and insulin resistance. Annu. Rev. Physiol. 72, 219–246 (2010).
Osborn, O. & Olefsky, J. M. The cellular and signaling networks linking the immune system and metabolism in disease. Nature Med. 18, 363–374 (2012).
Zhao, G. Q. et al. The receptors for mammalian sweet and umami taste. Cell 115, 255–266 (2003).
Urwyler, S. Allosteric modulation of family C G-protein-coupled receptors: from molecular insights to therapeutic perspectives. Pharmacol. Rev. 63, 59–126 (2011).
Nelson, G. et al. Mammalian sweet taste receptors. Cell 106, 381–390 (2001).
Li, X. et al. Human receptors for sweet and umami taste. Proc. Natl Acad. Sci. USA 99, 4692–4696 (2002).
Servant, G., Tachdjian, C., Li, X. & Karanewsky, D. S. The sweet taste of true synergy: positive allosteric modulation of the human sweet taste receptor. Trends Pharmacol. Sci. 32, 631–636 (2011). This is an excellent review on the development and properties of positive allosteric modulators of the sweet receptor.
Nie, Y., Vigues, S., Hobbs, J. R., Conn, G. L. & Munger, S. D. Distinct contributions of T1R2 and T1R3 taste receptor subunits to the detection of sweet stimuli. Curr. Biol. 15, 1948–1952 (2005).
Xu, H. et al. Different functional roles of T1R subunits in the heteromeric taste receptors. Proc. Natl Acad. Sci. USA 101, 14258–14263 (2004).
Jiang, P. et al. Identification of the cyclamate interaction site within the transmembrane domain of the human sweet taste receptor subunit T1R3. J. Biol. Chem. 280, 34296–34305 (2005).
Liu, B. et al. Molecular mechanism of species-dependent sweet taste toward artificial sweeteners. J. Neurosci. 31, 11070–11076 (2011).
Zhang, F. et al. Molecular mechanism for the umami taste synergism. Proc. Natl Acad. Sci. USA 105, 20930–20934 (2008).
Zhang, Y. et al. Coding of sweet, bitter, and umami tastes: different receptor cells sharing similar signaling pathways. Cell 112, 293–301 (2003).
Jang, H. J. et al. Gut-expressed gustducin and taste receptors regulate secretion of glucagon-like peptide-1. Proc. Natl Acad. Sci. USA 104, 15069–15074 (2007).
Kokrashvili, Z., Mosinger, B. & Margolskee, R. F. T1r3 and alpha-gustducin in gut regulate secretion of glucagon-like peptide-1. Ann. NY Acad. Sci. 1170, 91–94 (2009).
Gerspach, A. C., Steinert, R. E., Schonenberger, L., Graber-Maier, A. & Beglinger, C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am. J. Physiol. Endocrinol. Metab. 301, E317–E325 (2011).
Margolskee, R. F. et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proc. Natl Acad. Sci. USA 104, 15075–15080 (2007).
Reimann, F. et al. Glucose sensing in L-cells: a primary cell study. Cell. Metab. 8, 532–539 (2008).
Bezencon, C., le Coutre, J. & Damak, S. Taste-signaling proteins are coexpressed in solitary intestinal epithelial cells. Chem. Senses 32, 41–49 (2007).
Parker, H. E., Reimann, F. & Gribble, F. M. Molecular mechanisms underlying nutrient-stimulated incretin secretion. Expert. Rev. Mol. Med. 12, e1 (2010).
Kyriazis, G. A., Soundarapandian, M. M. & Tyrberg, B. Sweet taste receptor signaling in beta cells mediates fructose-induced potentiation of glucose-stimulated insulin secretion. Proc. Natl Acad. Sci. USA 109, E524–E532 (2012).
Shigemura, R. et al. Compositions comprising sweetness enhancers and methods of making them. WO Patent 2010/014813 (A2) (2010).
Servant, G. et al. Positive allosteric modulators of the human sweet taste receptor enhance sweet taste. Proc. Natl Acad. Sci. USA 107, 4746–4751 (2010).
Zhang, F. et al. Molecular mechanism of the sweet taste enhancers. Proc. Natl Acad. Sci. USA 107, 4752–4757 (2010).
Jiang, P. et al. Lactisole interacts with the transmembrane domains of human T1R3 to inhibit sweet taste. J. Biol. Chem. 280, 15238–15246 (2005).
Unger, R. H. The physiology of cellular liporegulation. Annu. Rev. Physiol. 65, 333–347 (2003).
Hara, T., Hirasawa, A., Ichimura, A., Kimura, I. & Tsujimoto, G. Free fatty acid receptors FFAR1 and GPR120 as novel therapeutic targets for metabolic disorders. J. Pharm. Sci. 100, 3594–3601 (2011).
Briscoe, C. P. et al. The orphan G protein-coupled receptor GPR40 is activated by medium and long chain fatty acids. J. Biol. Chem. 278, 11303–11311 (2003).
Itoh, Y. et al. Free fatty acids regulate insulin secretion from pancreatic β cells through GPR40. Nature 422, 173–176 (2003).
Kotarsky, K., Nilsson, N. E., Flodgren, E., Owman, C. & Olde, B. A human cell surface receptor activated by free fatty acids and thiazolidinedione drugs. Biochem. Biophys. Res. Commun. 301, 406–410 (2003).
Edfalk, S., Steneberg, P. & Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 57, 2280–2287 (2008).
Hirasawa, A. et al. Production and characterization of a monoclonal antibody against GPR40 (FFAR1; free fatty acid receptor 1). Biochem. Biophys. Res. Commun. 365, 22–28 (2008).
Cartoni, C. et al. Taste preference for fatty acids is mediated by GPR40 and GPR120. J. Neurosci. 30, 8376–8382 (2010). This paper provides the first description that FFA1 (GPR40) and GPR120 are involved in taste perception and mediate the taste of fatty acids.
Ma, D. et al. Expression of free fatty acid receptor GPR40 in the central nervous system of adult monkeys. Neurosci. Res. 58, 394–401 (2007).
Tomita, T. et al. Expression of the gene for a membrane-bound fatty acid receptor in the pancreas and islet cell tumours in humans: evidence for GPR40 expression in pancreatic beta cells and implications for insulin secretion. Diabetologia 49, 962–968 (2006).
Steneberg, P., Rubins, N., Bartoov-Shifman, R., Walker, M. D. & Edlund, H. The FFA receptor GPR40 links hyperinsulinemia, hepatic steatosis, and impaired glucose homeostasis in mouse. Cell. Metab. 1, 245–258 (2005).
Kebede, M. et al. The fatty acid receptor GPR40 plays a role in insulin secretion in vivo after high-fat feeding. Diabetes 57, 2432–2437 (2008).
Tan, C. P. et al. Selective small-molecule agonists of G protein-coupled receptor 40 promote glucose-dependent insulin secretion and reduce blood glucose in mice. Diabetes 57, 2211–2219 (2008).
Latour, M. G. et al. GPR40 is necessary but not sufficient for fatty acid stimulation of insulin secretion in vivo. Diabetes 56, 1087–1094 (2007).
Briscoe, C. P. et al. Pharmacological regulation of insulin secretion in MIN6 cells through the fatty acid receptor GPR40: identification of agonist and antagonist small molecules. Br. J. Pharmacol. 148, 619–628 (2006).
Song, F. et al. Synthesis and biological evaluation of 3-aryl-3-(4-phenoxy)-propionic acid as a novel series of G protein-coupled receptor 40 agonists. J. Med. Chem. 50, 2807–2817 (2007).
Nagasumi, K. et al. Overexpression of GPR40 in pancreatic β-cells augments glucose stimulated insulin secretion and improves glucose tolerance in normal and diabetic mice. Diabetes 58, 1067–1076 (2009).
Kebede, M. et al. Glucose activates free fatty acid receptor 1 gene transcription via phosphatidylinositol-3-kinase-dependent O-GlcNAcylation of pancreas-duodenum homeobox-1. Proc. Natl Acad. Sci. USA 109, 2376–2381 (2012).
Garrido, D. M. et al. Synthesis and activity of small molecule GPR40 agonists. Bioorg. Med. Chem. Lett. 16, 1840–1845 (2006).
Christiansen, E. et al. Structure–activity study of dihydrocinnamic acids and discovery of the potent FFA1 (GPR40) agonist TUG-469. ACS Med. Chem. Lett. 1, 345–349 (2010).
Christiansen, E. et al. Identification of a potent and selective free fatty acid receptor 1 (FFA1/GPR40) agonist with favorable physicochemical and in vitro ADME properties. J. Med. Chem. 54, 6691–6703 (2011).
Walsh, S. P. et al. 3-substituted 3-(4-aryloxyaryl)-propanoic acids as GPR40 agonists. Bioorg. Med. Chem. Lett. 21, 3390–3394 (2011).
Lin, D. C. et al. AMG 837: a novel GPR40/FFA1 agonist that enhances insulin secretion and lowers glucose levels in rodents. PLoS ONE 6, e27270 (2011).
Houze, J. B. et al. AMG 837: a potent, orally bioavailable GPR40 agonist. Bioorg. Med. Chem. Lett. 22, 1267–1270 (2012).
Tsujihata, Y. et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid. receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J. Pharmacol. Exp. Ther. 339, 228–237 (2011).
Sasaki, S. et al. Design, synthesis, and biological activity of potent and orally available G protein-coupled receptor 40 agonists. J. Med. Chem. 54, 1365–1378 (2011).
Negoro, N. et al. Identification of fused-ring alkanoic acids with improved pharmacokinetic profiles that act as G protein-coupled receptor 40/free fatty acid receptor 1 agonists. J. Med. Chem. 55, 1538–1552 (2012).
Negoro, N. et al. Optimization of (2,3-dihydro-1-benzofuran-3-yl)acetic acids: discovery of a non-free fatty acid-like, highly bioavailable G protein-coupled receptor 40/free fatty acid receptor 1 agonist as a glucose-dependent insulinotropic agent. J. Med. Chem. 55, 3960–3974 (2012).
Mikami, S. et al. Discovery of phenylpropanoic acid derivatives containing polar functionalities as potent and orally bioavailable G protein-coupled receptor 40 agonists for the treatment of type 2 diabetes. J. Med. Chem. 55, 3756–3776 (2012).
Burant, C. F. et al. TAK-875 versus placebo or glimepiride in type 2 diabetes mellitus: a Phase 2, randomised, double-blind, placebo-controlled trial. Lancet 379, 1403–1411 (2012). This is the first report on a Phase II clinical trial of a synthetic FFA1 agonist showing glucose-lowering effects that were comparable to those of a sulphonylurea but with less tendency to produce hypoglycaemia.
Stoddart, L. A., Brown, A. J. & Milligan, G. Uncovering the pharmacology of the G protein-coupled receptor GPR40: high apparent constitutive activity in guanosine 5′-O-(3-[35S]thio)triphosphate binding studies reflects binding of an endogenous agonist. Mol. Pharmacol. 71, 994–1005 (2007).
Smith, N. J., Stoddart, L. A., Devine, N. M., Jenkins, L. & Milligan, G. The action and mode of binding of thiazolidinedione ligands at free fatty acid receptor 1. J. Biol. Chem. 284, 17527–17539 (2009).
Zhou, C. et al. Discovery of 5-aryloxy-2,4-thiazolidinediones as potent GPR40 agonists. Bioorg. Med. Chem. Lett. 20, 1298–1301 (2010).
Hu, H. et al. A novel class of antagonists for the FFAs receptor GPR40. Biochem. Biophys. Res. Commun. 390, 557–563 (2009).
Tikhonova, I. G. et al. Discovery of novel agonists and antagonists of the free fatty acid receptor 1 (FFAR1) using virtual screening. J. Med. Chem. 51, 625–633 (2008).
Humphries, P. S. et al. Synthesis and SAR of 1,2,3,4-tetrahydroisoquinolin-1-ones as novel G-protein-coupled receptor 40 (GPR40) antagonists. Bioorg. Med. Chem. Lett. 19, 2400–2403 (2009).
Hirasawa, A. et al. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nature Med. 11, 90–94 (2005).
Moore, K., Zhang, Q., Murgolo, N., Hosted, T. & Duffy, R. Cloning, expression, and pharmacological characterization of the GPR120 free fatty acid receptor from cynomolgus monkey: comparison with human GPR120 splice variants. Comp. Biochem. Physiol. B. Biochem. Mol. Biol. 154, 419–426 (2009).
Galindo, M. M. et al. G protein-coupled receptors in human fat taste perception. Chem. Senses 37, 123–139 (2012).
Watson, S. J., Brown, A. J. & Holliday, N. Differential signalling by splice variants of the human free fatty acid receptor, GPR120. Mol. Pharmacol. 81, 631–642 (2012).
Gotoh, C. et al. The regulation of adipogenesis through GPR120. Biochem. Biophys. Res. Commun. 354, 591–597 (2007).
Oh Da, Y. et al. GPR120 is an omega-3 fatty acid receptor mediating potent anti-inflammatory and insulin-sensitizing effects. Cell 142, 687–698 (2010). This is the first description of GPR120 as a receptor for omega-3 fatty acids that mediates anti-inflammatory and insulin-sensitizing effects.
Miyauchi, S. et al. Distribution and regulation of protein expression of the free fatty acid receptor GPR120. Naunyn Schmiedebergs Arch. Pharmacol. 379, 427–434 (2009).
Talukdar, S., Olefsky, J. M. & Osborn, O. Targeting GPR120 and other fatty acid-sensing GPCRs ameliorates insulin resistance and inflammatory diseases. Trends Pharmacol. Sci. 32, 543–550 (2011).
Ichimura, A. et al. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature 483, 350–354 (2012). This is the first report showing that dysfunction of GPR120 promotes insulin resistance and obesity in mice and humans. This study also reports on a missense mutation in the human receptor, which interferes with GPR120-mediated signalling and is strongly correlated with an increased risk of developing obesity.
Sun, Q. et al. Structure–activity relationships of GPR120 agonists based on a docking simulation. Mol. Pharmacol. 78, 804–810 (2010).
Shimpukade, B., Hudson, B. D., Hovgaard, C. K., Milligan, G. & Ulven, T. Discovery of a potent and selective GPR120 agonist. J. Med. Chem. 55, 4511–4515 (2012).
Shi, D. F. et al. GPR120 receptor agonists and uses thereof. US Patent 2010/190831 (A1) (2010).
Shi, D. F. et al. GPR120 receptor agonists and uses thereof. WO Patent 2011/159297 (A1) (2011).
Shi, D. F. et al. GPR120 receptor agonists and uses thereof. US Patent 2011/313003 (A1) (2011).
Epple, R. et al. Thiazole derivatives as modulators of G protein-coupled receptors. WO Patent 2008103500 (A1) (2008).
Hashimoto, N. et al. Novel phenyl-isoxazol-3-ol derivative. US Patent 2010/130559 (2010).
Ishikawa, M. et al. Novel isoxazole derivative. EP Patent 2298750(A1) (2011).
Wang, J., Wu, X., Simonavicius, N., Tian, H. & Ling, L. Medium-chain fatty acids as ligands for orphan G protein-coupled receptor GPR84. J. Biol. Chem. 281, 34457–34464 (2006).
Bouchard, C., Page, J., Bedard, A., Tremblay, P. & Vallieres, L. G protein-coupled receptor 84, a microglia-associated protein expressed in neuroinflammatory conditions. Glia 55, 790–800 (2007).
Venkataraman, C. & Kuo, F. The G-protein coupled receptor, GPR84 regulates IL-4 production by T lymphocytes in response to CD3 crosslinking. Immunol. Lett. 101, 144–153 (2005).
Nagasaki, H. et al. Inflammatory changes in adipose tissue enhance expression of GPR84, a medium-chain fatty acid receptor: TNFα enhances GPR84 expression in adipocytes. FEBS Lett. 586, 368–372 (2012).
Hakak, Y. Unett, D.J., Gatlin, J., Liaw, C.W. & Inc, A.P. Human G protein-coupled receptor and modulators thereof for the treatment of atherosclerosis and atherosclerotic disease and for the treatment of conditions related to MCP-1 expression. WO Patent 2007/027661 (A2) (2007).
Topping, D. L. & Clifton, P. M. Short-chain fatty acids and human colonic function: roles of resistant starch and nonstarch polysaccharides. Physiol. Rev. 81, 1031–1064 (2001).
Brown, A. J. et al. The orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J. Biol. Chem. 278, 11312–11319 (2003).
Le Poul, E. et al. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J. Biol. Chem. 278, 25481–25489 (2003).
Nilsson, N. E., Kotarsky, K., Owman, C. & Olde, B. Identification of a free fatty acid receptor, FFA2R, expressed on leukocytes and activated by short-chain fatty acids. Biochem. Biophys. Res. Commun. 303, 1047–1052 (2003).
Hong, Y. H. et al. Acetate and propionate short chain fatty acids stimulate adipogenesis via GPCR43. Endocrinology 146, 5092–5099 (2005).
Xiong, Y. et al. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl Acad. Sci. USA 101, 1045–1050 (2004).
Zaibi, M. S. et al. Roles of GPR41 and GPR43 in leptin secretory responses of murine adipocytes to short chain fatty acids. FEBS Lett. 584, 2381–2386 (2010).
Kimura, I. et al. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc. Natl Acad. Sci. USA 108, 8030–8035 (2011).
Bjursell, M. et al. Improved glucose control and reduced body fat mass in free fatty acid receptor 2-deficient mice fed a high-fat diet. Am. J. Physiol. Endocrinol. Metab. 300, E211–E220 (2011).
Karaki, S. et al. Short-chain fatty acid receptor, GPR43, is expressed by enteroendocrine cells and mucosal mast cells in rat intestine. Cell Tissue Res. 324, 353–360 (2006).
Tazoe, H. et al. Expression of short-chain fatty acid receptor GPR41 in the human colon. Biomed. Res. 30, 149–156 (2009).
Tazoe, H. et al. Roles of short-chain fatty acids receptors, GPR41 and GPR43 on colonic functions. J. Physiol. Pharmacol. 59 (Suppl. 2), 251–262 (2008).
Samuel, B. S. et al. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc. Natl Acad. Sci. USA 105, 16767–16772 (2008).
Karaki, S. et al. Expression of the short-chain fatty acid receptor, GPR43, in the human colon. J. Mol. Histol. 39, 135–142 (2008).
Kaji, I., Karaki, S., Tanaka, R. & Kuwahara, A. Density distribution of free fatty acid receptor 2 (FFA2)-expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J. Mol. Histol. 42, 27–38 (2011).
Tolhurst, G. et al. Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 (2012).
Lin, H. V. et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 7, e35240 (2012).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009). This is the first report showing that FFA2 activation by short-chain fatty acids links diet and gastrointestinal bacterial metabolism with immune and inflammatory responses.
Senga, T. et al. LSSIG is a novel murine leukocyte-specific GPCR that is induced by the activation of STAT3. Blood 101, 1185–1187 (2003).
Vinolo, M. A. et al. SCFAs induce mouse neutrophil chemotaxis through the GPR43 receptor. PLoS ONE 6, e21205 (2011).
Sina, C. et al. G protein-coupled receptor 43 is essential for neutrophil recruitment during intestinal inflammation. J. Immunol. 183, 7514–7522 (2009).
Ge, H. et al. Activation of G protein-coupled receptor 43 in adipocytes leads to inhibition of lipolysis and suppression of plasma free fatty acids. Endocrinology 149, 4519–4526 (2008).
Wang, Y. et al. The first synthetic agonists of FFA2: discovery and SAR of phenylacetamides as allosteric modulators. Bioorg. Med. Chem. Lett. 20, 493–498 (2010).
Lee, T. et al. Identification and functional characterization of allosteric agonists for the G protein-coupled receptor FFA2. Mol. Pharmacol. 74, 1599–1609 (2008).
Schmidt, J. et al. Selective orthosteric free fatty acid receptor 2 (FFA2) agonists: identification of the structural and chemical requirements for selective activation of FFA2 versus FFA3. J. Biol. Chem. 286, 10628–10640 (2011).
Hoveyda, H., Zoute, L. & Lenoir, F. Novel compounds, method for use them and pharmaceutical composition containing them. WO Patent 2011/151436(A2) (2011).
Brantis, C. E., Ooms, F. & Bernard, J. Novel amino acid derivatives and their use as GPR43 receptor modulators. WO Patent 2011/092284(A1) (2011).
Cai, T. Q. et al. Role of GPR81 in lactate-mediated reduction of adipose lipolysis. Biochem. Biophys. Res. Commun. 377, 987–991 (2008).
Liu, C. et al. Lactate inhibits lipolysis in fat cells through activation of an orphan G-protein-coupled receptor, GPR81. J. Biol. Chem. 284, 2811–2822 (2009).
Taggart, A. K. et al. (d)-β-hydroxybutyrate inhibits adipocyte lipolysis via the nicotinic acid receptor PUMA-G. J. Biol. Chem. 280, 26649–26652 (2005).
Ahmed, K. et al. Deorphanization of GPR109B as a receptor for the beta-oxidation intermediate 3-OH-octanoic acid and its role in the regulation of lipolysis. J. Biol. Chem. 284, 21928–21933 (2009).
Offermanns, S. et al. International Union of Basic and Clinical Pharmacology. LXXXII: nomenclature and classification of hydroxy-carboxylic acid receptors (GPR81, GPR109A, and GPR109B). Pharmacol. Rev. 63, 269–290 (2011).
Ahmed, K. et al. An autocrine lactate loop mediates insulin-dependent inhibition of lipolysis through GPR81. Cell. Metab. 11, 311–319 (2010). This is the first report on the physiological role of GPR81 as a receptor for lactate in insulin-induced anti-lipolysis.
Tunaru, S. et al. PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nature Med. 9, 352–355 (2003).
Kostylina, G., Simon, D., Fey, M. F., Yousefi, S. & Simon, H. U. Neutrophil apoptosis mediated by nicotinic acid receptors (GPR109A). Cell Death Differ. 15, 134–142 (2008).
Tang, Y. et al. Enhancement of arachidonic acid signaling pathway by nicotinic acid receptor HM74A. Biochem. Biophys. Res. Commun. 345, 29–37 (2006).
Ge, H. et al. Elucidation of signaling and functional activities of an orphan GPCR, GPR81. J. Lipid Res. 49, 797–803 (2008).
Irukayama-Tomobe, Y. et al. Aromatic d-amino acids act as chemoattractant factors for human leukocytes through a G protein-coupled receptor, GPR109B. Proc. Natl Acad. Sci. USA 106, 3930–3934 (2009).
Soga, T. et al. Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 303, 364–369 (2003).
Wise, A. et al. Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 278, 9869–9874 (2003).
Richman, J. G. et al. Nicotinic acid receptor agonists differentially activate downstream effectors. J. Biol. Chem. 282, 18028–18036 (2007).
Benyó, Z., Gille, A., Bennett, C. L., Clausen, B. E. & Offermanns, S. Nicotinic acid-induced flushing is mediated by activation of epidermal Langerhans cells. Mol. Pharmacol. 70, 1844–1849 (2006).
Exton, J. H. Regulation of phosphoinositide phospholipases by hormones, neurotransmitters, and other agonists linked to G proteins. Annu. Rev. Pharmacol. Toxicol. 36, 481–509 (1996).
Jeninga, E. H. et al. Peroxisome proliferator-activated receptor γ regulates expression of the anti-lipolytic G-protein-coupled receptor 81 (GPR81/Gpr81). J. Biol. Chem. 284, 26385–26393 (2009).
Feingold, K. R., Moser, A., Shigenaga, J. K. & Grunfeld, C. Inflammation inhibits GPR81 expression in adipose tissue. Inflamm. Res. 60, 991–995 (2011).
Jansson, P. A., Larsson, A., Smith, U. & Lonnroth, P. Lactate release from the subcutaneous tissue in lean and obese men. J. Clin. Invest. 93, 240–246 (1994).
Qvisth, V., Hagstrom-Toft, E., Moberg, E., Sjoberg, S. & Bolinder, J. Lactate release from adipose tissue and skeletal muscle in vivo: defective insulin regulation in insulin-resistant obese women. Am. J. Physiol. Endocrinol. Metab. 292, E709–E714 (2007).
DiGirolamo, M., Newby, F. D. & Lovejoy, J. Lactate production in adipose tissue: a regulated function with extra-adipose implications. FASEB J. 6, 2405–2412 (1992).
Liu, C. et al. 3,5-dihydroxybenzoic acid, a specific agonist for HCA1, inhibits lipolysis in adipocytes. J. Pharmacol. Exp. Ther. 341, 794–801 (2012).
Marklund, M., Landberg, R., Anderson, A., Aman, P. & Kamal-Eldin, A. Alkylresorcinol metabolites in urine correlate with the intake of whole grains and cereal fibre in free-living Swedish adults. Br. J. Nutr. 3, 1–8 (2012).
Liu, C., Lovenberg, T. W. & Wu, J. GPR81-ligand complexes and their preparation and use. WO Patent 2008/063321 (A2) (2008).
Boatman, P. D. et al. 3H-imidazo[4,5-b]pyridin-5-ol derivatives useful in the treatment of GPR81 receptor disorders. WO Patent 2010/030360(A1) (2010).
Benyo, Z. et al. GPR109A (PUMA-G/HM74A) mediates nicotinic acid-induced flushing. J. Clin. Invest. 115, 3634–3640 (2005).
Maciejewski-Lenoir, D. et al. Langerhans cells release prostaglandin D2 in response to nicotinic acid. J. Invest. Dermatol. 126, 2637–2646 (2006).
Schaub, A., Futterer, A. & Pfeffer, K. PUMA-G, an IFN-γ-inducible gene in macrophages is a novel member of the seven transmembrane spanning receptor superfamily. Eur. J. Immunol. 31, 3714–3725 (2001).
Hanson, J. et al. Nicotinic acid- and monomethyl fumarate-induced flushing involves GPR109A expressed by keratinocytes and COX-2-dependent prostanoid formation in mice. J. Clin. Invest. 120, 2910–2919 (2010).
Thangaraju, M. et al. GPR109A is a G-protein-coupled receptor for the bacterial fermentation product butyrate and functions as a tumor suppressor in colon. Cancer Res. 69, 2826–2832 (2009).
Cresci, G. A., Thangaraju, M., Mellinger, J. D., Liu, K. & Ganapathy, V. Colonic gene expression in conventional and germ-free mice with a focus on the butyrate receptor GPR109A and the butyrate transporter SLC5A8. J. Gastrointest. Surg. 14, 449–461 (2010).
Martin, P. M. et al. Expression and localization of GPR109A (PUMA-G/HM74A) mRNA and protein in mammalian retinal pigment epithelium. Mol. Vis. 15, 362–372 (2009).
Gambhir, D. et al. GPR109A as an anti-inflammatory receptor in retinal pigment epithelial cells and its relevance to diabetic retinopathy. Invest. Ophthalmol. Vis. Sci. 53, 2208–2217 (2012).
Owen, O. E., Felig, P., Morgan, A. P., Wahren, J. & Cahill, G. F. Jr. Liver and kidney metabolism during prolonged starvation. J. Clin. Invest. 48, 574–583 (1969).
Senior, B. & Loridan, L. Direct regulatory effect of ketones on lipolysis and on glucose concentrations in man. Nature 219, 83–84 (1968).
Gille, A., Bodor, E. T., Ahmed, K. & Offermanns, S. Nicotinic acid: pharmacological effects and mechanisms of action. Annu. Rev. Pharmacol. Toxicol. 48, 79–106 (2008).
Brown, G. et al. Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B. N. Engl. J. Med. 323, 1289–1298 (1990).
Brown, B. G. et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N. Engl. J. Med. 345, 1583–1592 (2001).
Taylor, A. J. et al. Extended-release niacin or ezetimibe and carotid intima-media thickness. N. Engl. J. Med. 361, 2113–2122 (2009).
Canner, P. L. et al. Fifteen year mortality in coronary drug project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 8, 1245–1255 (1986).
Carlson, L. A. & Rosenhamer, G. Reduction of mortality in the Stockholm Ischaemic Heart Disease Secondary Prevention Study by combined treatment with clofibrate and nicotinic acid. Acta Med. Scand. 223, 405–418 (1988).
The Coronary Drug Project Research Group. Clofibrate and niacin in coronary heart disease. JAMA 231, 360–381 (1975).
Blankenhorn, D. H. et al. Beneficial effects of combined colestipol-niacin therapy on coronary atherosclerosis and coronary venous bypass grafts. JAMA 257, 3233–3240 (1987).
Digby, J. E. et al. Anti-inflammatory effects of nicotinic acid in human monocytes are mediated by GPR109A dependent mechanisms. Arterioscler. Thromb. Vasc. Biol. 32, 669–676 (2012).
Blankenhorn, D. H. et al. Effects of colestipol-niacin therapy on human femoral atherosclerosis. Circulation 83, 438–447 (1991).
Lee, J. M. et al. Effects of high-dose modified-release nicotinic acid on atherosclerosis and vascular function: a randomized, placebo-controlled, magnetic resonance imaging study. J. Am. Coll. Cardiol. 54, 1787–1794 (2009).
Whitney, E. J. et al. A randomized trial of a strategy for increasing high-density lipoprotein cholesterol levels: effects on progression of coronary heart disease and clinical events. Ann. Intern. Med. 142, 95–104 (2005).
Boden, W. E. et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Nicholls, S. J. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve. Clin. J. Med. 79, 38–43 (2012).
Bloomgarden, Z. & Handelsman, Y. Did AIM-HIGH aim too low? J. Diabetes 3, 1–2 (2011).
Carlson, L. A. Studies on the effect of nicotinic acid on catecholamine stimulated lipolysis in adipose tissue in vitro. Acta Med. Scand. 173, 719–722 (1963).
Joy, T. & Hegele, R. A. Is raising HDL a futile strategy for atheroprotection? Nature Rev. Drug. Discov. 7, 143–155 (2008).
Hernandez, M., Wright, S. D. & Cai, T. Q. Critical role of cholesterol ester transfer protein in nicotinic acid-mediated HDL elevation in mice. Biochem. Biophys. Res. Commun. 355, 1075–1080 (2007).
van der Hoorn, J. W. et al. Niacin increases HDL by reducing hepatic expression and plasma levels of cholesteryl ester transfer protein in APOE*3Leiden.CETP mice. Arterioscler. Thromb. Vasc. Biol. 28, 2016–2022 (2008).
Kontush, A. & Chapman, M. J. Antiatherogenic small, dense HDL — guardian angel of the arterial wall? Nature Clin. Pract. Cardiovasc. Med. 3, 144–153 (2006).
Offermanns, S. The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol. Sci. 27, 384–390 (2006).
Bodor, E. T. & Offermanns, S. Nicotinic acid: an old drug with a promising future. Br. J. Pharmacol. 153 (Suppl. 1), S68–S75 (2008).
Li, X., Millar, J. S., Brownell, N., Briand, F. & Rader, D. J. Modulation of HDL metabolism by the niacin receptor GPR109A in mouse hepatocytes. Biochem. Pharmacol. 80, 1450–1457 (2010).
Kamanna, V. S. & Kashyap, M. L. Mechanism of action of niacin. Am. J. Cardiol. 101, 20B–26B (2008).
Lai, E. et al. Effects of a niacin receptor partial agonist, MK-0354, on plasma free fatty acids, lipids, and cutaneous flushing in humans. J. Clin. Lipidol. 2, 375–383 (2008).
Boatman, P. D. et al. (1aR,5aR)1a, 3,5,5a-tetrahydro-1H-2,3-diaza-cyclopropa[a]pentalene-4-carboxylic acid: a potent GPR109a agonist that lowers free fatty acids in humans. J. Med. Chem. 55, 3644–3666 (2012). This is the first report on a Phase II clinical study of a full HCA 2 (GPR109A) agonist showing anti-lipolytic effects but no increase in HDL cholesterol plasma levels.
Lukasova, M., Hanson, J., Tunaru, S. & Offermanns, S. Nicotinic acid (niacin): new lipid-independent mechanisms of action and therapeutic potentials. Trends Pharmacol. Sci. 32, 700–707 (2011).
Lukasova, M., Malaval, C., Gille, A., Kero, J. & Offermanns, S. Nicotinic acid inhibits progression of atherosclerosis in mice through its receptor GPR109A expressed by immune cells. J. Clin. Invest. 121, 1163–1173 (2011).
Wu, B. J. et al. Evidence that niacin inhibits acute vascular inflammation and improves endothelial dysfunction independent of changes in plasma lipids. Arterioscler. Thromb. Vasc. Biol. 30, 968–975 (2010). References 184 and 185 are the first studies to show the lipid-independent beneficial cardiovascular effects of nicotinic acid. Some of these effects appear to be mediated by HCA 2.
Plaisance, E. P. et al. Niacin stimulates adiponectin secretion through the GPR109A receptor. Am. J. Physiol. Endocrinol. Metab. 296, E549–E558 (2009).
Westphal, S., Borucki, K., Taneva, E., Makarova, R. & Luley, C. Extended-release niacin raises adiponectin and leptin. Atherosclerosis 193, 361–365 (2007).
Ingersoll, M. A. et al. Niacin inhibits skin dendritic cell mobilization in a GPR109A independent manner but has no impact on monocyte trafficking in atherosclerosis. Immunobiology 217, 548–557 (2011).
Holzhauser, E. et al. Nicotinic acid has anti-atherogenic and anti-inflammatory properties on advanced atherosclerotic lesions independent of its lipid-modifying capabilities. J. Cardiovasc. Pharmacol. 57, 447–454 (2011).
Kamanna, V. S., Ganji, S. H. & Kashyap, M. L. The mechanism and mitigation of niacin-induced flushing. Int. J. Clin. Pract. 63, 1369–1377 (2009).
Cheng, K. et al. Antagonism of the prostaglandin D2 receptor 1 suppresses nicotinic acid-induced vasodilation in mice and humans. Proc. Natl Acad. Sci. USA 103, 6682–6687 (2006).
Paolini, J. F. et al. Effects of laropiprant on nicotinic acid-induced flushing in patients with dyslipidemia. Am. J. Cardiol. 101, 625–630 (2008).
Walters, R. W. et al. β-arrestin1 mediates nicotinic acid-induced flushing, but not its antilipolytic effect, in mice. J. Clin. Invest. 119, 1312–1321 (2009).
Rajagopal, S., Rajagopal, K. & Lefkowitz, R. J. Teaching old receptors new tricks: biasing seven-transmembrane receptors. Nature Rev. Drug Discov. 9, 373–386 (2010).
Tang, H., Lu, J. Y., Zheng, X., Yang, Y. & Reagan, J. D. The psoriasis drug monomethylfumarate is a potent nicotinic acid receptor agonist. Biochem. Biophys. Res. Commun. 375, 562–565 (2008). This is the first report on fumaric acid esters as ligands of HCA 2.
Papadopoulou, A., D'Souza, M., Kappos, L. & Yaldizli, O. Dimethyl fumarate for multiple sclerosis. Expert. Opin. Investig. Drugs 19, 1603–1612 (2010).
[No authors listed]. Trial watch: Phase III success for Biogen's oral multiple sclerosis therapy. Nature Rev. Drug Discov. 10, 404 (2011).
Zhang, J. et al. Niaspan treatment improves neurological functional recovery in experimental autoimmune encephalomyelitis mice. Neurobiol. Dis. 32, 273–280 (2008).
Schilling, S., Goelz, S., Linker, R., Luehder, F. & Gold, R. Fumaric acid esters are effective in chronic experimental autoimmune encephalomyelitis and suppress macrophage infiltration. Clin. Exp. Immunol. 145, 101–107 (2006).
Fuccella, L. M. et al. Inhibition of lipolysis by nicotinic acid and by acipimox. Clin. Pharmacol. Ther. 28, 790–795 (1980).
Cayen, M. N., Kallai-Sanfacon, M. A., Dubuc, J., Greselin, E. & Dvornik, D. Evaluation of the lipid-lowering activity of AY-25712 in rats. Atherosclerosis 45, 267–279 (1982).
Soudijn, W., van Wijngaarden, I. & IJzerman, A. P. Nicotinic acid receptor subtypes and their ligands. Med. Res. Rev. 27, 417–433 (2007).
Semple, G., Boatman, P. D. & Richman, J. G. Recent progress in the discovery of niacin receptor agonists. Curr. Opin. Drug Discov. Devel. 10, 452–459 (2007).
van Herk, T. et al. Pyrazole derivatives as partial agonists for the nicotinic acid receptor. J. Med. Chem. 46, 3945–3951 (2003).
Gharbaoui, T. et al. Agonist lead identification for the high affinity niacin receptor GPR109a. Bioorg. Med. Chem. Lett. 17, 4914–4919 (2007).
Skinner, P. J. et al. Fluorinated pyrazole acids are agonists of the high affinity niacin receptor GPR109a. Bioorg. Med. Chem. Lett. 17, 5620–5623 (2007).
Semple, G. et al. 3-(1H-tetrazol-5-yl)-1,4,5,6-tetrahydro-cyclopentapyrazole (MK-0354): a partial agonist of the nicotinic acid receptor, G-protein coupled receptor 109a, with antilipolytic but no vasodilatory activity in mice. J. Med. Chem. 51, 5101–5108 (2008).
Boatman, P. D. et al. Potent tricyclic pyrazole tetrazole agonists of the nicotinic acid receptor (GPR109a). Bioorg. Med. Chem. Lett. 20, 2797–2800 (2010).
Imbriglio, J. E. et al. GPR109a agonists. Part 1: 5-alkyl and 5-aryl-pyrazole-tetrazoles as agonists of the human orphan G-protein coupled receptor GPR109a. Bioorg. Med. Chem. Lett. 19, 2121–2124 (2009).
Schmidt, D. et al. Pyrazole acids as niacin receptor agonists for the treatment of dyslipidemia. Bioorg. Med. Chem. Lett. 19, 4768–4772 (2009).
Imbriglio, J. E. et al. GPR109a agonists. Part 2: pyrazole-acids as agonists of the human orphan G-protein coupled receptor GPR109a. Bioorg. Med. Chem. Lett. 20, 4472–4474 (2010).
Ren, N. et al. Phenolic acids suppress adipocyte lipolysis via activation of the nicotinic acid receptor GPR109A (HM74a/PUMA-G). J. Lipid Res. 50, 908–914 (2009).
van Veldhoven, J. P. et al. Structure–activity relationships of trans-substituted-propenoic acid derivatives on the nicotinic acid receptor HCA2 (GPR109A). Bioorg. Med. Chem. Lett. 21, 2736–2739 (2011).
Shen, H. C. et al. Discovery of biaryl anthranilides as full agonists for the high affinity niacin receptor. J. Med. Chem. 50, 6303–6306 (2007).
Shen, H. C. et al. Discovery of orally bioavailable and novel urea agonists of the high affinity niacin receptor GPR109A. Bioorg. Med. Chem. Lett. 17, 6723–6728 (2007).
Schmidt, D. et al. Anthranilic acid replacements in a niacin receptor agonist. Bioorg. Med. Chem. Lett. 20, 3426–3430 (2010).
Raghavan, S. et al. Tetrahydro anthranilic acid as a surrogate for anthranilic acid: application to the discovery of potent niacin receptor agonists. Bioorg. Med. Chem. Lett. 18, 3163–3167 (2008).
Ding, F. X. et al. Discovery of pyrazolyl propionyl cyclohexenamide derivatives as full agonists for the high affinity niacin receptor GPR109A. Bioorg. Med. Chem. Lett. 20, 3372–3375 (2010).
Shen, H. C. et al. Discovery of a biaryl cyclohexene carboxylic acid (MK-6892): a potent and selective high affinity niacin receptor full agonist with reduced flushing profiles in animals as a preclinical candidate. J. Med. Chem. 53, 2666–2670 (2010).
Peters, J. U. et al. Pyrido pyrimidinones as selective agonists of the high affinity niacin receptor GPR109A: optimization of in vitro activity. Bioorg. Med. Chem. Lett. 20, 5426–5430 (2010).
Shen, H. C. et al. Discovery of pyrazolopyrimidines as the first class of allosteric agonists for the high affinity nicotinic acid receptor GPR109A. Bioorg. Med. Chem. Lett. 18, 4948–4951 (2008).
Blad, C. C. et al. Novel 3,6,7-substituted pyrazolopyrimidines as positive allosteric modulators for the hydroxycarboxylic acid receptor 2 (GPR109A). J. Med. Chem. 55, 3563–3567 (2012).
Boatman, P. D., Richman, J. G. & Semple, G. Nicotinic acid receptor agonists. J. Med. Chem. 51, 7653–7662 (2008).
Martres, P. HM74a agonists: will they be the new generation of nicotinic acid? Curr. Top. Med. Chem. 9, 428–435 (2009).
Shen, H. C. Acyl hydroxypyrazoles as novel agonists for high-affinity nicotinic acid receptor GPR109A: WO2008051403. Expert Opin. Ther. Pat. 19, 1149–1155 (2009).
Shen, H. C. & Colletti, S. L. Novel patent publications on high-affinity nicotinic acid receptor agonists. Expert Opin. Ther. Pat. 19, 957–967 (2009).
Nomura, H., Nielsen, B. W. & Matsushima, K. Molecular cloning of cDNAs encoding a LD78 receptor and putative leukocyte chemotactic peptide receptors. Int. Immunol. 5, 1239–1249 (1993).
Yousefi, S. Cooper, P. R., Mueck, B., Potter, S. L. & Jarai, G. cDNA representational difference analysis of human neutrophils stimulated by GM-CSF. Biochem. Biophys. Res. Commun. 277, 401–409 (2000).
Costa, C. G. et al. Simultaneous analysis of plasma free fatty acids and their 3-hydroxy analogs in fatty acid beta-oxidation disorders. Clin. Chem. 44, 463–471 (1998).
Jones, P. M., Tjoa, S., Fennessey, P. V., Goodman, S. I. & Bennett, M. J. Addition of quantitative 3-hydroxy-octadecanoic acid to the stable isotope gas chromatography-mass spectrometry method for measuring 3-hydroxy fatty acids. Clin. Chem. 48, 176–179 (2002).
Ahmed, K., Tunaru, S. & Offermanns, S. GPR109A, GPR109B and GPR81, a family of hydroxy-carboxylic acid receptors. Trends Pharmacol. Sci. 30, 557–562 (2009).
Mandrika, I., Petrovska, R. & Klovins, J. Evidence for constitutive dimerization of niacin receptor subtypes. Biochem. Biophys. Res. Commun. 395, 281–287 (2010).
Mahboubi, K. et al. Triglyceride modulation by acifran analogs: activity towards the niacin high and low affinity G protein-coupled receptors HM74A and HM74. Biochem. Biophys. Res. Commun. 340, 482–490 (2006).
Jung, J. K. et al. Analogues of acifran: agonists of the high and low affinity niacin receptors, GPR109a and GPR109b. J. Med. Chem. 50, 1445–1448 (2007).
Semple, G. et al. 1-alkyl-benzotriazole-5-carboxylic acids are highly selective agonists of the human orphan G-protein-coupled receptor GPR109b. J. Med. Chem. 49, 1227–1230 (2006).
Skinner, P. J. et al. 3-nitro-4-amino benzoic acids and 6-amino nicotinic acids are highly selective agonists of GPR109b. Bioorg. Med. Chem. Lett. 17, 6619–6622 (2007).
Skinner, P. J. et al. 5-N,N-disubstituted 5-aminopyrazole-3-carboxylic acids are highly potent agonists of GPR109b. Bioorg. Med. Chem. Lett. 19, 4207–4209 (2009).
Ariza, A. C., Deen, P. M. T. & Robben, J. H. The succinate receptor as a novel therapeutic target for oxidative and metabolic stress-related conditions. Front. Endocrinol. 3, 22 (2012). This is an excellent review on the physiological and pathological functions of SUCNR1 and its potential as a pharmacological target.
He, W. et al. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429, 188–193 (2004).
Toma, I. et al. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J. Clin. Invest. 118, 2526–2534 (2008).
Sapieha, P. et al. The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nature Med. 14, 1067–1076 (2008).
Rubic, T. et al. Triggering the succinate receptor GPR91 on dendritic cells enhances immunity. Nature Immunol. 9, 1261–1269 (2008).
Vargas, S. L., Toma, I., Kang, J. J., Meer, E. J. & Peti-Peterdi, J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J. Am. Soc. Nephrol. 20, 1002–1011 (2009).
Amisten, S., Braun, O. O., Bengtsson, A. & Erlinge, D. Gene expression profiling for the identification of G-protein coupled receptors in human platelets. Thromb. Res. 122, 47–57 (2008).
Regard, J. B., Sato, I. T. & Coughlin, S. R. Anatomical profiling of G protein-coupled receptor expression. Cell 135, 561–571 (2008).
Peti-Peterdi, J. High glucose and renin release: the role of succinate and GPR91. Kidney Int. 78, 1214–1217 (2010).
Robben, J. H. et al. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int. 76, 1258–1267 (2009).
Bhuniya, D. et al. Discovery of a potent and selective small molecule hGPR91 antagonist. Bioorg. Med. Chem. Lett. 21, 3596–3602 (2011).
Wittenberger, T. et al. GPR99, a new G protein-coupled receptor with homology to a new subgroup of nucleotide receptors. BMC Genomics 3, 17 (2002).
Qi, A. D., Harden, T. K. & Nicholas, R. A. GPR80/99, proposed to be the P2Y(15) receptor activated by adenosine and AMP, is not a P2Y receptor. Purinerg. Signal. 1, 67–74 (2004).
Wagner, B. M., Donnarumma, F., Wintersteiger, R., Windischhofer, W. & Leis, H. J. Simultaneous quantitative determination of alpha-ketoglutaric acid and 5-hydroxymethylfurfural in human plasma by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 396, 2629–2637 (2010).
Ahmadian, M., Wang, Y. & Sul, H. S. Lipolysis in adipocytes. Int. J. Biochem. Cell Biol. 42, 555–559 (2010).
Zechner, R. et al. FAT SIGNALS — lipases and lipolysis in lipid metabolism and signaling. Cell. Metab. 15, 279–291 (2012).
Tunaru, S., Lattig, J., Kero, J., Krause, G. & Offermanns, S. Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G). Mol. Pharmacol. 68, 1271–1280 (2005).
Acknowledgements
The authors wish to thank S. Hümmer for excellent secretarial help with the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Glossary
- Enteroendocrine cells
-
Specialized endocrine cells of the gastrointestinal tract that are in contact with the gut lumen and secrete various hormones such as the incretins glucagon-like peptide 1 and gastric inhibitory peptide.
- Citric acid cycle
-
Also known as the Krebs cycle; the process by which organisms aerobically generate energy through the oxidation of acetate.
- Protomers
-
Units of an oligomeric protein; a protein dimer consists of two protomers.
- Orthosteric agonists
-
Ligands that bind to the receptor at the same site as the endogenous ligand of the receptor.
- Allosteric agonists
-
Ligands that bind to the receptor at a site different to that of the endogenous ligand (or ligands).
- Glucagon-like peptide 1
-
(GLP1). A peptide hormone produced by enteroendocrine cells that stimulates insulin secretion from pancreatic β-cells.
- Post-prandial
-
After a meal.
- Incretins
-
A group of gastrointestinal hormones, including glucagon-like peptide 1 and gastric inhibitory peptide, that regulate insulin secretion.
- L-cells
-
A subgroup of enteroendocrine cells that produce glucagon-like peptide 1 and peptide YY with an amino-terminal tyrosine amide.
- Ketogenesis
-
The process by which fatty acids, which are degraded in the liver, are further metabolized into ketone bodies.
- Prostaglandin D2 receptor
-
A G protein-coupled receptor that is activated by prostaglandin D2.
- Apolipoprotein E
-
A particular apolipoprotein that is found on chylomicrons and intermediate-density lipoproteins. Mice lacking the gene encoding apolipoprotein E are prone to developing atherosclerosis.
- β-oxidation
-
A process (occurring in mitochondria) by which fatty acids are broken down to acetyl-CoA.
- Macula densa cells
-
Specialized cells lining the wall of the distal tubulus of the kidney at the point where the distal tubulus comes into contact with its parent glomerulus.
Rights and permissions
About this article
Cite this article
Blad, C., Tang, C. & Offermanns, S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov 11, 603–619 (2012). https://doi.org/10.1038/nrd3777
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3777
This article is cited by
-
Ketosis prevents abdominal aortic aneurysm rupture through C–C chemokine receptor type 2 downregulation and enhanced extracellular matrix balance
Scientific Reports (2024)
-
The mechanism of oleic acid inhibiting platelet activation stimulated by collagen
Cell Communication and Signaling (2023)
-
A comprehensive molecular profiling approach reveals metabolic alterations that steer bone tissue regeneration
Communications Biology (2023)
-
Gut microbiome dysregulation drives bone damage in broiler tibial dyschondroplasia by disrupting glucose homeostasis
npj Biofilms and Microbiomes (2023)
-
The Promise of Niacin in Neurology
Neurotherapeutics (2023)