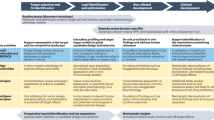
Key Points
-
Metabolism of drugs can generate metabolites that are chemically reactive towards cellular molecules and have the potential to alter biological function and initiate serious adverse drug reactions.
-
This Review details methods for the detection of chemically reactive metabolites (CRMs), and outlines the current industrial and academic knowledge about structural alerts.
-
The physiological response to bioactivation is discussed in the context of the toxicological response and how hypersensitivity reactions may occur.
-
We also discuss the management of CRMs during drug development, taking into account whether the currently used CRM decision trees are relevant to the challenges posed in drug development.
-
Earlier iteration between medicinal chemistry and drug metabolism can eliminate perceived reactive metabolite-mediated chemical liabilities without comprising pharmacological activity or the need for extensive safety evaluation beyond standard practices.
-
Finally, we address CRM-related decision-making based on minimal data (avoidance strategy) and how to make decisions based on covalent binding and other data. The implications for drug regulation are outlined.
Abstract
The normal metabolism of drugs can generate metabolites that have intrinsic chemical reactivity towards cellular molecules, and therefore have the potential to alter biological function and initiate serious adverse drug reactions. Here, we present an assessment of the current approaches used for the evaluation of chemically reactive metabolites. We also describe how these approaches are being used within the pharmaceutical industry to assess and minimize the potential of drug candidates to cause toxicity. At early stages of drug discovery, iteration between medicinal chemistry and drug metabolism can eliminate perceived reactive metabolite-mediated chemical liabilities without compromising pharmacological activity or the need for extensive safety evaluation beyond standard practices. In the future, reactive metabolite evaluation may also be useful during clinical development for improving clinical risk assessment and risk management. Currently, there remains a huge gap in our understanding of the basic mechanisms that underlie chemical stress-mediated adverse reactions in humans. This Review summarizes our views on this complex topic, and includes insights into practices considered by the pharmaceutical industry.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Lasser, K. E. et al. Timing of new black box warnings and withdrawals for prescription medications. JAMA 287, 2215–2220 (2002).
Ostapowicz, G. et al. Results of a prospective study of acute liver failure at 17 tertiary care centers in the United States. Ann. Intern. Med. 137, 947–954 (2002).
Adams, D. H., Ju, C., Ramaiah, S. K., Uetrecht, J. & Jaeschke, H. Mechanisms of immune-mediated liver injury. Toxicol. Sci. 115, 307–321 (2010).
Li, J. & Uetrecht, J. P. The danger hypothesis applied to idiosyncratic drug reactions. Handb. Exp. Pharmacol. 196, 493–509 (2010).
Park, B. K., Kitteringham, N. R., Maggs, J. L., Pirmohamed, M. & Williams, D. P. The role of metabolic activation in drug-induced hepatotoxicity. Annu. Rev. Pharmacol. Toxicol. 45, 177–202 (2005).
Erve, J. C. Chemical toxicology: reactive intermediates and their role in pharmacology and toxicology. Expert Opin. Drug Metab. Toxicol. 2, 923–946 (2006).
Guengerich, F. P. & MacDonald, J. S. Applying mechanisms of chemical toxicity to predict drug safety. Chem. Res. Toxicol. 20, 344–369 (2007).
Kalgutkar, A. S. & Soglia, J. R. Minimising the potential for metabolic activation in drug discovery. Expert Opin. Drug Metab. Toxicol. 1, 91–142 (2005).
Nelson, S. D. Structure toxicity relationships — how useful are they in predicting toxicities of new drugs? Adv. Exp. Med. Biol. 500, 33–43 (2001).
Walgren, J. L., Mitchell, M. D. & Thompson, D. C. Role of metabolism in drug-induced idiosyncratic hepatotoxicity. Crit. Rev. Toxicol. 35, 325–361 (2005).
Tirmenstein, M. A. & Nelson, S. D. Subcellular binding and effects on calcium homeostasis produced by acetaminophen and a nonhepatotoxic regioisomer, 3′-hydroxyacetanilide, in mouse liver. J. Biol. Chem. 264, 9814–9819 (1989).
Liebler, D. C. Protein damage by reactive electrophiles: targets and consequences. Chem. Res. Toxicol. 21, 117–128 (2008).
Kenna, J. G. in Drug Induced Liver Disease (eds Kaplowitz, N., & DeLeve, L. D.) 465–484 (Marcel Dekker, New York, 2007).
Obach, R. S., Kalgutkar, A. S., Soglia, J. R. & Zhao, S. X. Can in vitro metabolism-dependent covalent binding data in liver microsomes distinguish hepatotoxic from nonhepatotoxic drugs? An analysis of 18 drugs with consideration of intrinsic clearance and daily dose. Chem. Res. Toxicol. 21, 1814–1822 (2008).
Coles, B. et al. The spontaneous and enzymatic reaction of N-acetyl-p-benzoquinonimine with glutathione: a stopped-flow kinetic study. Arch. Biochem. Biophys. 264, 253–260 (1988).
Tingle, M. D., Mahmud, R., Maggs, J. L., Pirmohamed, M. & Park, B. K. Comparison of the metabolism and toxicity of dapsone in rat, mouse and man. J. Pharmacol. Exp. Ther. 283, 817–823 (1997).
Nakayama, S. et al. A zone classification system for risk assessment of idiosyncratic drug toxicity using daily dose and covalent binding. Drug Metab. Dispos. 37, 1970–1977 (2009).
Usui, T., Mise, M., Hashizume, T., Yabuki, M. & Komuro, S. Evaluation of the potential for drug-induced liver injury based on in vitro covalent binding to human liver proteins. Drug Metab. Dispos. 37, 2383–2392 (2009).
de Rooij, B. M., Boogaard, P. J., Commandeur, J. N., van Sittert, N. J. & Vermeulen, N. P. Allylmercapturic acid as urinary biomarker of human exposure to allyl chloride. Occup. Environ. Med. 54, 653–661 (1997).
Nelson, E. B. The pharmacology and toxicology of meta-substituted acetanilide I: acute toxicity of 3-hydroxyacetanilide in mice. Res. Commun. Chem. Pathol. Pharmacol. 28, 447–456 (1980).
Bauman, J. N. et al. Can in vitro metabolism-dependent covalent binding data distinguish hepatotoxic from nonhepatotoxic drugs? An analysis using human hepatocytes and liver S-9 fraction. Chem. Res. Toxicol. 22, 332–340 (2009).
Mallal, S. et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet 359, 727–732 (2002).
Chessman, D. et al. Human leukocyte antigen class I-restricted activation of CD8+ T cells provides the immunogenetic basis of a systemic drug hypersensitivity. Immunity 28, 822–832 (2008).
Evans, D. C., Watt, A. P., Nicoll-Griffith, D. A. & Baillie, T. A. Drug–protein adducts: an industry perspective on minimizing the potential for drug bioactivation in drug discovery and development. Chem. Res. Toxicol. 17, 3–16 (2004).
Smith, D. A. & Obach, R. S. Metabolites in safety testing (MIST): considerations of mechanisms of toxicity with dose, abundance, and duration of treatment. Chem. Res. Toxicol. 22, 267–279 (2009).
Smith, D. A., Obach, R. S., Williams, D. P. & Park, B. K. Clearing the MIST (metabolites in safety testing) of time: the impact of duration of administration on drug metabolite toxicity. Chem. Biol. Interact. 179, 60–67 (2009).
Kalgutkar, A. S. et al. A comprehensive listing of bioactivation pathways of organic functional groups. Curr. Drug Metab. 6, 161–225 (2005).
Uetrecht, J. Prediction of a new drug's potential to cause idiosyncratic reactions. Curr. Opin. Drug Discov. Devel. 4, 55–59 (2001).
Day, S. H. et al. A semi-automated method for measuring the potential for protein covalent binding in drug discovery. J. Pharmacol. Toxicol. Methods 52, 278–285 (2005).
Baillie, T. A. Metabolism and toxicity of drugs. Two decades of progress in industrial drug metabolism. Chem. Res. Toxicol. 21, 129–137 (2008).
Bateman, K. P. et al. MSE with mass defect filtering for in vitro and in vivo metabolite identification. Rapid Commun. Mass Spectrom. 21, 1485–1496 (2007).
Fontana, E., Dansette, P. M. & Poli, S. M. Cytochrome p450 enzymes mechanism based inhibitors: common sub-structures and reactivity. Curr. Drug Metab. 6, 413–454 (2005).
Kalgutkar, A. S., Obach, R. S. & Maurer, T. S. Mechanism-based inactivation of cytochrome P450 enzymes: chemical mechanisms, structure–activity relationships and relationship to clinical drug–drug interactions and idiosyncratic adverse drug reactions. Curr. Drug Metab. 8, 407–447 (2007).
Danciu, T. E. & Whitman, M. Oxidative stress drives disulfide bond formation between basic helix–loop–helix transcription factors. J. Cell Biochem. 109, 417–424 (2010).
Jacobs, A. T. & Marnett, L. J. Heat shock factor 1 attenuates 4-hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J. Biol. Chem. 282, 33412–33420 (2007).
Lieu, H. T. et al. Reg2 inactivation increases sensitivity to Fas hepatotoxicity and delays liver regeneration post-hepatectomy in mice. Hepatology 44, 1452–1464 (2006).
Bateman, K. P. et al. Detection of covalent adducts to cytochrome P450 3A4 using liquid chromatography mass spectrometry. Chem. Res. Toxicol. 17, 1356–1361 (2004).
Fang, J., Koen, Y. M. & Hanzlik, R. P. Bioinformatic analysis of xenobiotic reactive metabolite target proteins and their interacting partners. BMC Chem. Biol. 9, 5 (2009).
Hanzlik, R. P., Koen, Y. M., Theertham, B., Dong, Y. & Fang, J. The reactive metabolite target protein database (TPDB) — a web-accessible resource. BMC Bioinformatics 8, 95 (2007).
Gan, J., Harper, T. W., Hsueh, M. M., Qu, Q. & Humphreys, W. G. Dansyl glutathione as a trapping agent for the quantitative estimation and identification of reactive metabolites. Chem. Res. Toxicol. 18, 896–903 (2005).
Yan, Z., Maher, N., Torres, R. & Huebert, N. Use of a trapping agent for simultaneous capturing and high-throughput screening of both “soft” and “hard” reactive metabolites. Anal. Chem. 79, 4206–4214 (2007).
Ma, L., Wen, B., Ruan, Q. & Zhu, M. Rapid screening of glutathione-trapped reactive metabolites by linear ion trap mass spectrometry with isotope pattern-dependent scanning and postacquisition data mining. Chem. Res. Toxicol. 21, 1477–1483 (2008).
Ma, X. & Chan, E. C. Fluorescence-based liver microsomal assay for screening of pharmaceutical reactive metabolites using a glutathione conjugated 96-well plate. Bioconjug. Chem. 21, 46–55 (2010).
Fontana, R. J. et al. Acute liver failure associated with prolonged use of bromfenac leading to liver transplantation. The Acute Liver Failure Study Group. Liver Transpl. Surg. 5, 480–484 (1999).
Hunter, E. B., Johnston, P. E., Tanner, G., Pinson, C. W. & Awad, J. A. Bromfenac (Duract)-associated hepatic failure requiring liver transplantation. Am. J. Gastroenterol. 94, 2299–2301 (1999).
Moses, P. L. et al. Severe hepatotoxicity associated with bromfenac sodium. Am. J. Gastroenterol. 94, 1393–1396 (1999).
Goldkind, L. & Laine, L. A systematic review of NSAIDs withdrawn from the market due to hepatotoxicity: lessons learned from the bromfenac experience. Pharmacoepidemiol Drug Saf. 15, 213–220 (2006).
Miyanaga, M. et al. Effect of bromfenac ophthalmic solution on ocular inflammation following cataract surgery. Acta Ophthalmol. 87, 300–305 (2009).
Bakke, O. M., Manocchia, M., de Abajo, F., Kaitin, K. I. & Lasagna, L. Drug safety discontinuations in the United Kingdom, the United States, and Spain from 1974 through 1993: a regulatory perspective. Clin. Pharmacol. Ther. 58, 108–117 (1995).
Berson, A. et al. Inhibition by nilutamide of the mitochondrial respiratory chain and ATP formation. Possible contribution to the adverse effects of this antiandrogen. J. Pharmacol. Exp. Ther. 270, 167–176 (1994).
Wen, B. et al. Comparison of in vitro bioactivation of flutamide and its cyano analogue: evidence for reductive activation by human NADPH: cytochrome P450 reductase. Chem. Res. Toxicol. 21, 2393–2406 (2008).
Schellhammer, P. F. et al. Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group. Urology 50, 330–336 (1997).
Coe, K. J. et al. Comparison of the cytotoxicity of the nitroaromatic drug flutamide to its cyano analogue in the hepatocyte cell line TAMH: evidence for complex I inhibition and mitochondrial dysfunction using toxicogenomic screening. Chem. Res. Toxicol. 20, 1277–1290 (2007).
Bailey, M. J. & Dickinson, R. G. Acyl glucuronide reactivity in perspective: biological consequences. Chem. Biol. Interact. 145, 117–137 (2003).
Boelsterli, U. A. Nimesulide and hepatic adverse effects: roles of reactive metabolites and host factors. Int. J. Clin. Pract. 128, 30–36 (2002).
Boelsterli, U. A. Mechanisms of NSAID-induced hepatotoxicity: focus on nimesulide. Drug Saf. 25, 633–648 (2002).
Skonberg, C., Olsen, J., Madsen, K. G., Hansen, S. H. & Grillo, M. P. Metabolic activation of carboxylic acids. Expert Opin. Drug Metab. Toxicol. 4, 425–438 (2008).
Boelsterli, U. A. Xenobiotic acyl glucuronides and acyl CoA thioesters as protein-reactive metabolites with the potential to cause idiosyncratic drug reactions. Curr. Drug Metab. 3, 439–450 (2002).
Kitteringham, N. R. et al. Hepatocellular response to chemical stress in CD-1 mice: induction of early genes and γ-glutamylcysteine synthetase. Hepatology 32, 321–333 (2000).
Dambach, D. M., Durham, S. K., Laskin, J. D. & Laskin, D. L. Distinct roles of NF-κB p50 in the regulation of acetaminophen-induced inflammatory mediator production and hepatotoxicity. Toxicol. Appl. Pharmacol. 211, 157–165 (2006).
Xu, Z. et al. Liver-specific inactivation of the Nrf1 gene in adult mouse leads to nonalcoholic steatohepatitis and hepatic neoplasia. Proc. Natl Acad. Sci. USA 102, 4120–4125 (2005).
Copple, I. M., Goldring, C. E., Kitteringham, N. R. & Park, B. K. The keap1-nrf2 cellular defense pathway: mechanisms of regulation and role in protection against drug-induced toxicity. Handb. Exp. Pharmacol. 196, 233–266 (2010).
Chevillard, G., Nouhi, Z., Anna, D., Paquet, M. & Blank, V. Nrf3-deficient mice are not protected against acute lung and adipose tissue damages induced by butylated hydroxytoluene. FEBS Lett. 584, 923–928.
Cheng, J., Ma, X., Krausz, K. W., Idle, J. R. & Gonzalez, F. J. Rifampicin-activated human pregnane X receptor and CYP3A4 induction enhance acetaminophen-induced toxicity. Drug Metab. Dispos. 37, 1611–1621 (2009).
Grosch, S., Fritz, G. & Kaina, B. Apurinic endonuclease (Ref-1) is induced in mammalian cells by oxidative stress and involved in clastogenic adaptation. Cancer Res. 58, 4410–4416 (1998).
Bykov, V. J., Lambert, J. M., Hainaut, P. & Wiman, K. G. Mutant p53 rescue and modulation of p53 redox state. Cell Cycle 8, 2509–2517 (2009).
Rzymski, T., Milani, M., Singleton, D. C. & Harris, A. L. Role of ATF4 in regulation of autophagy and resistance to drugs and hypoxia. Cell Cycle 8, 3838–3847 (2009).
Pessler-Cohen, D. et al. GLUT4 repression in response to oxidative stress is associated with reciprocal alterations in C/EBP α and δ isoforms in 3T3-L1 adipocytes. Arch. Physiol. Biochem. 112, 3–12 (2006).
Stamper, B. D., Bammler, T. K., Beyer, R. P., Farin, F. M. & Nelson, S. D. Differential regulation of mitogen-activated protein kinase pathways by acetaminophen and its nonhepatotoxic regioisomer 3′-hydroxyacetanilide in TAMH cells. Toxicol. Sci. 116, 164–173 (2010).
McCubrey, J. A., Lahair, M. M. & Franklin, R. A. Reactive oxygen species-induced activation of the MAP kinase signaling pathways. Antioxid. Redox Signal. 8, 1775–1789 (2006).
Copple, I. M. et al. The hepatotoxic metabolite of acetaminophen directly activates the Keap1-Nrf2 cell defense system. Hepatology 48, 1292–1301 (2008).
Dinkova-Kostova, A. T. et al. Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl Acad. Sci. USA 99, 11908–11913 (2002).
Hong, F., Sekhar, K. R., Freeman, M. L. & Liebler, D. C. Specific patterns of electrophile adduction trigger Keap1 ubiquitination and Nrf2 activation. J. Biol. Chem. 280, 31768–31775 (2005).
Itoh, K., Mimura, J. & Yamamoto, M. Discovery of the negative regulator of Nrf2, Keap1: A historical overview. Antioxid. Redox Signal. 13, 1665–1678 (2010).
Goldring, C. E. et al. Activation of hepatic Nrf2 in vivo by acetaminophen in CD-1 mice. Hepatology 39, 1267–1276 (2004).
Nassif, A. et al. Toxic epidermal necrolysis: effector cells are drug-specific cytotoxic T cells. J. Allergy Clin. Immunol. 114, 1209–1215 (2004).
Farrell, J. et al. Characterization of sulfamethoxazole and sulfamethoxazole metabolite-specific T-cell responses in animals and humans. J. Pharmacol. Exp. Ther. 306, 229–237 (2003).
Schnyder, B. et al. Recognition of sulfamethoxazole and its reactive metabolites by drug-specific CD4+ T cells from allergic individuals. J. Immunol. 164, 6647–6654 (2000).
Sanderson, J. P. et al. Sulfamethoxazole and its metabolite nitroso sulfamethoxazole stimulate dendritic cell costimulatory signaling. J. Immunol. 178, 5533–5542 (2007).
Lavergne, S. N. et al. Drug metabolite-specific lymphocyte responses in sulfamethoxazole allergic patients with cystic fibrosis. Chem. Res. Toxicol. 23, 1009–1011 (2010).
Park, B. K., Pirmohamed, M. & Kitteringham, N. R. Role of drug disposition in drug hypersensitivity: a chemical, molecular, and clinical perspective. Chem. Res. Toxicol. 11, 969–988 (1998).
Kitteringham, N. R., Kenna, J. G. & Park, B. K. Detection of autoantibodies directed against human hepatic endoplasmic-reticulum in sera from patients with halothane-associated hepatitis. Br. J. Clin. Pharmacol. 40, 379–386 (1995).
Cheng, L., You, Q., Yin, H., Holt, M. P. & Ju, C. Involvement of natural killer T cells in halothane-induced liver injury in mice. Biochem. Pharmacol. 80, 255–261 (2010).
You, Q., Cheng, L. & Ju, C. Generation of T cell responses targeting the reactive metabolite of halothane in mice. Toxicol. Lett. 194, 79–85 (2010).
Maria, V. A. & Victorino, R. M. Immunological investigation in hepatic drug reactions. Clin. Exp. Allergy 28 (Suppl. 4), 71–77 (1998).
Aithal, G. P. et al. Hepatic adducts, circulating antibodies, and cytokine polymorphisms in patients with diclofenac hepatotoxicity. Hepatology 39, 1430–1440 (2004).
Daly, A. K. et al. Genetic susceptibility to diclofenac-induced hepatotoxicity: contribution of UGT2B7, CYP2C8, and ABCC2 genotypes. Gastroenterology 132, 272–281 (2007).
Hanzlik, R. P., Fang, J. & Koen, Y. M. Filling and mining the reactive metabolite target protein database. Chem. Biol. Interact. 179, 38–44 (2009).
Nakamura, Y. et al. Redox regulation of glutathione S-transferase induction by benzyl isothiocyanate: correlation of enzyme induction with the formation of reactive oxygen intermediates. Cancer Res. 60, 219–225 (2000).
Acknowledgements
The authors would like to acknowledge the Medical Research Council (MRC) and The Association of British Pharmaceutical Companies (ABPI). The Merck retrospective analysis was conducted in-house with a crossfunctional team led by D.N.-G. with recognition to J. Monroe and F. Sistare for curating the safety assessment data, members of DMPK for CB data and the sponsorship of L. Shipley, J. Harrelson and D. Dean. An industrial–academic workshop that was held in Liverpool, UK, on 13 and 14 April 2010 formed the initiative for the development of this manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
Scott Obach is an employee and shareholder of Pfizer — a company that is engaged in the discovery, development and sale of pharmaceuticals for profit.
Philip Routledge is head of the section of Pharmacology, Therapeutics & Toxicology at Cardiff University, UK, which has a clinical lectureship that is unconditionally part-funded by AstraZeneca under the Association for British Pharmaceutical Industries (ABPI) Clinical Pharmacology Training Scheme and part-funded by the Welsh Assembly Government.
Deborah Nicoll-Griffith is an employee of Merck & Co.
All other authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Park, B., Boobis, A., Clarke, S. et al. Managing the challenge of chemically reactive metabolites in drug development. Nat Rev Drug Discov 10, 292–306 (2011). https://doi.org/10.1038/nrd3408
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd3408
This article is cited by
-
What’s been Hapten-ing over the last 88 years?
Medicinal Chemistry Research (2023)
-
Interactions of selected cardiovascular active natural compounds with CXCR4 and CXCR7 receptors: a molecular docking, molecular dynamics, and pharmacokinetic/toxicity prediction study
BMC Complementary Medicine and Therapies (2022)
-
In-line formation and identification of toxic reductive metabolites of aristolochic acid using electrochemistry mass spectrometry coupling
Analytical and Bioanalytical Chemistry (2022)
-
Determination of Daphnetin and its 8-O-Methylated Metabolite in Rat Plasma by UFLC-MS/MS: Application to a Pharmacokinetic Study
Chromatographia (2022)
-
Antiparkinsonian activity of Cucurbita pepo seeds along with possible underlying mechanism
Metabolic Brain Disease (2021)