Abstract
In the era of cytotoxic therapies, tumor regression has rarely been observed in phase I trials and randomized controlled trials have usually been required to demonstrate modest improvements over prevailing standards of care. In the era of effective targeted therapies, drugs such as vemurafenib and crizotinib have demonstrated convincing efficacy in early clinical testing, raising the question of whether randomized phase III trials are necessary and feasible before drug approval. Since 1992, the FDA has approved a number of drugs without data from confirmatory clinical trials as part of the accelerated approval process. While this initiative has largely been successful in bringing effective drugs to the market more quickly, there is much to be learned from case studies of drugs, such as gefitinib, which subsequently failed to gain full approval. In this Review, we use a number of historical examples to make the case that it may be reasonable to consider foregoing randomized phase III trials for certain drugs before drug approval. We explore the consequences (both good and bad) of foregoing randomized phase III trials and propose criteria that might be used to select drugs for consideration of such an approach.
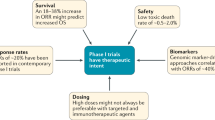
Key Points
-
There have been significant advances in the development of targeted anticancer therapies that are evident even in early-phase clinical trials
-
It may be reasonable to forego randomized phase III trials for those drugs that demonstrate substantial activity before phase III, particularly for diseases with a high unmet medical need
-
The consequences of foregoing randomized phase III trials must be recognized, most notably the risk of approving drugs without definitive evidence of efficacy and a limited knowledge of safety
-
Additional research and/or expert discussion is needed, along with guidance from regulatory agencies, to better define the criteria for selecting drugs to forego randomized phase III trials before approval
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
US National Library of Medicine. ClinicalTrials.gov [online].
Flaherty, K. et al. Phase I study of PLX4032: Proof of concept for V600E BRAF mutation as a therapeutic target in human cancer [abstract]. J. Clin. Oncol. 27 (Suppl. 15), 9000 (2009).
Flaherty, K. T. et al. Inhibition of mutated, activated BRAF in metastatic melanoma. N. Engl. J. Med. 363, 809–819 (2010).
US National Library of Medicine. ClinicalTrials.gov [online].
Roche. Roche personalized investigational medicine shows survival benefit in advanced skin cancer [online], (2011).
Chapman, P. B. et al. Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N. Engl. J. Med. 364, 2507–2516 (2011).
FDA. Highlights of prescribing information for Zelboraf (vemurafenib) [online], (2011).
Harmon, A. New drugs stir debate on rules of clinical trials. The New York Times (New York) A1 (19 Sept 2010).
Miller, F. G. & Joffe, S. Equipoise and the dilemma of randomized clinical trials. N. Engl. J. Med. 364, 476–480 (2011).
Miller, F. G. & Joffe, S. Balancing access and evaluation in the approval of new cancer drugs. JAMA 305, 2345–2346 (2011).
Joffe, S. & Miller, F. G. Equipoise: Asking the right questions for clinical trial design. Nat. Rev. Clin. Oncol. (in press)
PhRMA. More than 800 medicines and vaccines in testing offer hope in the fight against cancer [online], (2009).
Huang, M. E. et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood 72, 567–572 (1988).
Soignet, S. L. et al. Complete remission after treatment of acute promyelocytic leukemia with arsenic trioxide. N. Engl. J. Med. 339, 1341–1348 (1998).
Maloney, D. G. et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood 90, 2188–2195 (1997).
Kantarjian, H. et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N. Engl. J. Med. 346, 645–652 (2002).
Demetri, G. D. et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N. Engl. J. Med. 347, 472–480 (2002).
Von Hoff, D. D. et al. Inhibition of the hedgehog pathway in advanced basal-cell carcinoma. N. Engl. J. Med. 361, 1164–1172 (2009).
Kwak, E. L. et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N. Engl. J. Med. 363, 1693–1703 (2010).
Fukuoka, M. et al. Multi-institutional randomized phase II trial of gefitinib for previously treated patients with advanced non-small-cell lung cancer (The IDEAL 1 Trial). J. Clin. Oncol. 21, 2237–2246 (2003).
Kris, M. G. et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer: a randomized trial. JAMA 290, 2149–2158 (2003).
Giaccone, G. et al. Gefitinib in combination with gemcitabine and cisplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 1. J. Clin. Oncol. 22, 777–784 (2004).
Herbst, R. S. et al. Gefitinib in combination with paclitaxel and carboplatin in advanced non-small-cell lung cancer: a phase III trial—INTACT 2. J. Clin. Oncol. 22, 785–794 (2004).
Thatcher, N. et al. Gefitinib plus best supportive care in previously treated patients with refractory advanced non-small-cell lung cancer: results from a randomised, placebo-controlled, multicentre study (Iressa Survival Evaluation in Lung Cancer). Lancet 366, 1527–1537 (2005).
Tamura, K. & Fukuoka, M. Gefitinib in non-small cell lung cancer. Expert Opin. Pharmacother. 6, 985–993 (2005).
Sanford, M. & Scott, L. J. Gefitinib: a review of its use in the treatment of locally advanced/metastatic non-small cell lung cancer. Drugs 69, 2303–2328 (2009).
Sequist, L. V., Bell, D. W., Lynch, T. J. & Haber, D. A. Molecular predictors of response to epidermal growth factor receptor antagonists in non-small-cell lung cancer. J. Clin. Oncol. 25, 587–595 (2007).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Maemondo, M. et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N. Engl. J. Med. 362, 2380–2388 (2010).
Keedy, V. L. et al. American Society of Clinical Oncology provisional clinical opinion: epidermal growth factor receptor (EGFR) mutation testing for patients with advanced non-small-cell lung cancer considering first-line EGFR tyrosine kinase inhibitor therapy. J. Clin. Oncol. 29, 2121–2127 (2011).
Department of Health and Human Services. New drug, antibiotic, and biological drug product regulations; accelerated approval—FDA. Final rule. Fed. Regist. 57, 58942–58960 (1992).
Johnson, J. R. et al. Accelerated approval of oncology products: the food and drug administration experience. J. Natl. Cancer Inst. 103, 636–644 (2011).
Food and Drug Administration Oncologic Drugs Advisory Committee. Transcript of the 8 February meeting, [online] (2011).
Tsimberidou, A. M., Braiteh, F., Stewart, D. J. & Kurzrock, R. Ultimate fate of oncology drugs approved by the US Food and Drug Administration without a randomized trial. J. Clin. Oncol. 27, 6243–6250 (2009).
Sievers, E. L. et al. Efficacy and safety of gemtuzumab ozogamicin in patients with CD33-positive acute myeloid leukemia in first relapse. J. Clin. Oncol. 19, 3244–3254 (2001).
FDA. Pfizer voluntarily withdraws cancer treatment Mylotarg from U. S. market [online], (2010).
Sharma, M. R., Stadler, W. M. & Ratain, M. J. Randomized phase II trials: a long-term investment with promising returns. J. Natl. Cancer Inst. 103, 1093–1100 (2011).
FDA. Guidance for industry: clinical trial endpoints for the approval of cancer drugs and biologics [online], (2007).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007).
Motzer, R. J. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124 (2007).
Motzer, R. J. et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372, 449–456 (2008).
Escudier, B. et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 370, 2103–2111 (2007).
Rini, B. I. et al. Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J. Clin. Oncol. 26, 5422–5428 (2008).
Sternberg, C. N. et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J. Clin. Oncol. 28, 1061–1068 (2010).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Van Cutsem, E. et al. Open-label phase III trial of panitumumab plus best supportive care compared with best supportive care alone in patients with chemotherapy-refractory metastatic colorectal cancer. J. Clin. Oncol. 25, 1658–1664 (2007).
Tang, P. A., Bentzen, S. M., Chen, E. X. & Siu, L. L. Surrogate end points for median overall survival in metastatic colorectal cancer: literature-based analysis from 39 randomized controlled trials of first-line chemotherapy. J. Clin. Oncol. 25, 4562–4568 (2007).
Buyse, M. et al. Progression-free survival is a surrogate for survival in advanced colorectal cancer. J. Clin. Oncol. 25, 5218–5224 (2007).
Lara, P. N. Jr. et al. Disease control rate at 8 weeks predicts clinical benefit in advanced non-small-cell lung cancer: results from Southwest Oncology Group randomized trials. J. Clin. Oncol. 26, 463–467 (2008).
Halabi, S. et al. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J. Clin. Oncol. 27, 2766–2771 (2009).
Heng, D. Y. et al. Progression-free survival as a predictor of overall survival in metastatic renal cell carcinoma treated with contemporary targeted therapy. Cancer 117, 2637–2642 (2011).
Cheng, S. K., Dietrich, M. S. & Dilts, D. M. A sense of urgency: evaluating the link between clinical trial development time and the accrual performance of cancer therapy evaluation program (NCI-CTEP) sponsored studies. Clin. Cancer Res. 16, 5557–5563 (2010).
Hamilton, E. P., Lyman, G. H. & Peppercorn, J. Availability of experimental therapy outside oncology randomized clinical trials in the United States. J. Clin. Oncol. 28, 5067–5073 (2010).
Schilsky, R. L. Accrual to cancer clinical trials in the era of molecular medicine. Sci. Transl. Med. 3, 75cm9 (2011).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357, 2666–2676 (2007).
D'Agostino, R. B. Sr . Changing end points in breast-cancer drug approval—the Avastin story. N. Engl. J. Med. 365, e2 (2011).
Online reference. http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2008/125085s091ltr.pdf (2008).
Food and Drug Administration Oncologic Drugs Advisory Committee. Transcript of the 20 July meeting [online], (2010).
O'Shaughnessy, J. et al. Iniparib plus chemotherapy in metastatic triple-negative breast cancer. N. Engl. J. Med. 364, 205–214 (2011).
Anders, C. K. et al. Poly(ADP-Ribose) polymerase inhibition: “targeted” therapy for triple-negative breast cancer. Clin. Cancer Res. 16, 4702–4710 (2010).
Pollack, A. Promising results in stomach and breast cancer drugs. The New York Times (New York) (1 June 2009).
O'Shaughnessy, J. et al. A randomized phase III study of iniparib (BSI-201) in combination with gemcitabine/carboplatin (G/C) in metastatic triple-negative breast cancer (TNBC) [abstract]. J. Clin. Oncol. 29 (Suppl. 15), a1007 (2011).
Richey, E. A. et al. Accelerated approval of cancer drugs: improved access to therapeutic breakthroughs or early release of unsafe and ineffective drugs? J. Clin. Oncol. 27, 4398–4405 (2009).
Psaty, B. M. & Prentice, R. L. Minimizing bias in randomized trials: the importance of blinding. JAMA 304, 793–794 (2010).
Burzykowski, T. et al. Evaluation of tumor response, disease control, progression-free survival, and time to progression as potential surrogate end points in metastatic breast cancer. J. Clin. Oncol. 26, 1987–1992 (2008).
Cohen, M. H. et al. Approval summary for imatinib mesylate capsules in the treatment of chronic myelogenous leukemia. Clin. Cancer Res. 8, 935–942 (2002).
Inoue, L. Y., Thall, P. F. & Berry, D. A. Seamlessly expanding a randomized phase II trial to phase III. Biometrics 58, 823–831 (2002).
Korn, E. L. & Freidlin, B. Outcome—adaptive randomization: is it useful? J. Clin. Oncol. 29, 771–776 (2011).
Berry, D. A. Adaptive clinical trials in oncology. Nat. Rev. Clin. Oncol. doi:10.1038/nrclinonc.2011.165 (2011).
Acknowledgements
This work was supported by a training grant from the National Institutes of Health for Clinical Therapeutics, T32GM007019. The funders did not have any involvement in the design of this Review; the collection, analysis, and interpretation of the data; the writing of the manuscript; or the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Contributions
M. R. Sharma completed the research of data for the article and wrote the manuscript. Both authors made a substantial contribution to discussion of the content and to editing and revising the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
R. L. Schilsky declares that he is a consultant for and owns shared from Foundation Medicine and that he is a consultant for Universal Oncology. M. R. Sharma declares no competing interests.
Rights and permissions
About this article
Cite this article
Sharma, M., Schilsky, R. Role of randomized phase III trials in an era of effective targeted therapies. Nat Rev Clin Oncol 9, 208–214 (2012). https://doi.org/10.1038/nrclinonc.2011.190
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2011.190
This article is cited by
-
Do Early Phase Oncology Trials Predict Clinical Efficacy in Subsequent Biomarker-Enriched Phase III Randomized Trials?
Targeted Oncology (2022)
-
Molecular imaging and molecular diagnostics: two sides of the same coin?
European Journal of Nuclear Medicine and Molecular Imaging (2018)
-
Quantitative assessment of pancreatic cancer precursor lesions in IHC-stained tissue with a tissue image analysis platform
Laboratory Investigation (2016)
-
Bayesian Two-Stage Biomarker-Based Adaptive Design for Targeted Therapy Development
Statistics in Biosciences (2016)
-
Implementing personalized cancer genomics in clinical trials
Nature Reviews Drug Discovery (2013)