Abstract
Despite their inherent selectivity, targeted therapies such as tyrosine kinase inhibitors (TKIs) can cause unusual adverse effects. Sunitinib and sorafenib are multitargeted TKIs that have been demonstrated to induce hypothyroidism and thyroid dysfunction. Retrospective studies indicate that sunitinib can induce hypothyroidism in 53–85% of patients, and in prospective studies this complication has been reported in 36–71% of patients. Sorafenib has been reported to be responsible for hypothyroidism in 18% of patients with metastatic renal-cell carcinoma. Furthermore, imatinib and sunitinib seem to increase the requirement of levothyroxine in hypothyroid patients. The management of thyroid dysfunction and possible related symptoms, such as fatigue, represents a challenge to oncologists. We propose a diagnostic and therapeutic algorithm for the management of TKI-related hypothyroidism. Prospective trials are needed to define the incidence of overt and subclinical hypothyroidism and thyroid dysfunction during therapy with sunitinib, sorafenib and potentially other TKIs. The safety and efficacy, and optimal dosing and timing of starting replacement therapy in patients affected by TKI-related hypothyroidism need accurate appraisal and should be evaluated prospectively in appropriately designed trials.
Key Points
-
The reported incidence of sunitinib-induced hypothyroidism is 53–85% and 36–46% in retrospective or prospective studies, respectively, and 18% in patients treated with sorafenib
-
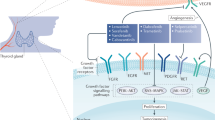
Mechanisms of hypothyroidism induced by TKIs include drug-induced atrophy of the thyroid through inhibition of its vascularization, drug-induced thyroiditis, reduced synthesis of thyroid hormones, progressive depletion of the thyroid's functional reserve and inhibition of the thyroidal iodine uptake
-
Whether TKI-related hypothyroidism is mediated by inhibition of the VEGF pathway or of other molecular pathways is unknown
-
In patients treated with sunitinib or sorafenib, routine thyroid function testing at baseline, and measurement of serum thyroid stimulating hormone level on day 1 at the start of every new treatment cycle is recommended
-
Levothyroxine is the standard treatment for overt hypothyroidism and is recommended in some patients with subclinical hypothyroidism; overt or subclinical hypothyroidism per se does not justify the withdrawal of TKI therapy
-
Thyroid function test should be included in routine toxicity assessment of TKIs under clinical evaluation; however, the clinical relevance of early diagnosis of hypothyroidism in patients with TKIs is still controversial
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Lacouture, M. E. Mechanisms of cutaneous toxicities to EGFR inhibitors. Nat. Rev. Cancer 6, 806–812 (2006).
Ross, D. S. Serum thyroid-stimulating hormone measurement for assessment of thyroid function and disease. Endocrinol. Metab. Clin. North. Am. 30, 245–264 (2001).
Vanderpump, M. P. et al. The incidence of thyroid disorders in the community: a twenty-year follow-up of the Whickham Survey. Clin. Endocrinol. (Oxf.) 43, 55–68 (1995).
Roberts, C. G. & Ladenson, P. W. Hypothyroidism. Lancet 363, 793–803 (2004).
Parle, J. V., Franklyn, J. A., Cross, K. W., Jones, S. C. & Sheppard, M. C. Prevalence and follow-up of abnormal thyrotrophin (TSH) concentrations in the elderly in the United Kingdom. Clin. Endocrinol. (Oxf.) 34, 77–83 (1991).
Robuschi, G., Safran, M., Braverman, L. E., Gnudi, A. & Roti, E. Hypothyroidism in the elderly. Endocr. Rev. 8, 142–153 (1987).
Hollowell, J. G. et al. Serum TSH T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J. Clin. Endocrinol. Metab. 87, 489–499 (2002).
Heymann, R. & Brent, G. A. Rapid progression from subclinical to symptomatic overt hypothyroidism. Endocr. Pract. 11, 115–119 (2005).
Yeung, S. C., Chiu, A. C., Vassilopoulou-Sellin, R. & Gagel, R. F. The endocrine effects of nonhormonal antineoplastic therapy. Endocr. Rev. 19, 144–172 (1998).
Stuart, N. S., Woodroffe, C. M., Grundy, R. & Cullen, M. H. Long-term toxicity of chemotherapy for testicular cancer—the cost of cure. Br. J. Cancer 61, 479–484 (1990).
Sutcliffe, S. B., Chapman, R. & Wrigley, P. F. Cyclical combination chemotherapy and thyroid function in patients with advanced Hodgkin's disease. Med. Pediatr. Oncol. 9, 439–448 (1981).
Mandac, J. C., Chaudhry, S., Sherman, K. E. & Tomer, Y. The clinical and physiological spectrum of interferon-alpha induced thyroiditis: toward a new classification. Hepatology 43, 661–672 (2006).
Vassilopoulou-Sellin, R. Endocrine effects of cytokines. Oncology (Williston Park) 8, 43–46 (1994).
Atkins, M. B. et al. Hypothyroidism after treatment with interleukin-2 and lymphokine-activated killer cells. N. Engl. J. Med. 318, 1557–1563 (1988).
Franzke, A. et al. Autoimmunity resulting from cytokine treatment predicts long-term survival in patients with metastatic renal cell cancer. J. Clin. Oncol. 17, 529–533 (1999).
Weijl, N. I. et al. Hypothyroidism during immunotherapy with interleukin-2 is associated with antithyroid antibodies and response to treatment. J. Clin. Oncol. 11, 1376–1383 (1993).
Krouse, R. S. et al. Thyroid dysfunction in 281 patients with metastatic melanoma or renal carcinoma treated with interleukin-2 alone. J. Immunother. Emphasis Tumor Immunol. 18, 272–278 (1995).
Badros, A. Z. et al. Hypothyroidism in patients with multiple myeloma following treatment with thalidomide. Am. J. Med. 112, 412–413 (2002).
de Savary, N., Lee, R. & Vaidya, B. Severe hypothyroidism after thalidomide treatment. J. R. Soc. Med. 97, 443 (2004).
Stein, E. M. & Rivera, C. Transient thyroiditis after treatment with lenalidomide in a patient with metastatic renal cell carcinoma. Thyroid 17, 681–683 (2007).
Sherman, S. I. et al. Central hypothyroidism associated with retinoid X receptor-selective ligands. N. Engl. J. Med. 340, 1075–1079 (1999).
Hancock, S. L., McDougall, I. R. & Constine, L. S. Thyroid abnormalities after therapeutic external radiation. Int. J. Radiat. Oncol. Biol. Phys. 31, 1165–1170 (1995).
Loeffler, J. S., Tarbell, N. J., Garber, J. R. & Mauch, P. The development of Graves' disease following radiation therapy in Hodgkin's disease. Int. J. Radiat. Oncol. Biol. Phys. 14, 175–178 (1988).
Petersen, M., Keeling, C. A. & McDougall, I. R. Hyperthyroidism with low radioiodine uptake after head and neck irradiation for Hodgkin's disease. J. Nucl. Med. 30, 255–257 (1989).
de Groot, J. W., Zonnenberg, B. A., Plukker, J. T., van Der Graaf, W. T. & Links, T. P. Imatinib induces hypothyroidism in patients receiving levothyroxine. Clin. Pharmacol. Ther. 78, 433–438 (2005).
Rini, B. I. et al. Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl Cancer Inst. 99, 81–83 (2007).
Wong, E. et al. Sunitinib induces hypothyroidism in advanced cancer patients and may inhibit thyroid peroxidase activity. Thyroid 17, 351–355 (2007).
de Groot, J. W., Links, T. P. & van der Graaf, W. T. Tyrosine kinase inhibitors causing hypothyroidism in a patient on levothyroxine. Ann. Oncol. 17, 1719–1720 (2006).
Desai, J. et al. Hypothyroidism after sunitinib treatment for patients with gastrointestinal stromal tumors. Ann. Intern. Med. 145, 660–664 (2006).
Feldman, D. R., Martorella, A. J., Robbins, R. J. & Motzer, R. J. Re: hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl Cancer Inst. 99, 974–975 (2007).
Schoeffski, P. et al. Sunitinib-related thyroid dysfunction: a single-center retrospective and prospective evaluation. J. Clin. Oncol. 24 (Suppl 18), 3092 (2006).
Mannavola, D. et al. A novel tyrosine kinase inhibitor, sunitinib, induces transient hypothyroidism by blocking iodine uptake. J. Clin. Endocrinol. Metab. 92, 3531–3534 (2007).
Tamaskar, I. et al. Thyroid function test abnormalities in patients with metastatic renal cell carcinoma treated with sorafenib. Ann. Oncol. 19, 265–268 (2008).
Curran, P. G. & DeGroot, L. J. The effect of hepatic enzyme-inducing drugs on thyroid hormones and the thyroid gland. Endocr. Rev. 12, 135–150 (1991).
Surks, M. I. & Sievert, R. Drugs and thyroid function. N. Engl. J. Med. 333, 1688–1694 (1995).
Schröder-van der Elst, J. P. et al. Effects of 5, 5'-diphenylhydantoin on the metabolic pathway of thyroid hormone in rats. Eur. J. Endocrinol. 136, 324–329 (1997).
De Luca, F. et al. Changes in thyroid function tests induced by 2 month carbamazepine treatment in L-thyroxine-substituted hypothyroid children. Eur. J. Pediatr. 145, 77–79 (1986).
Takasu, N., Takara, M. & Komiya, I. Rifampin-induced hypothyroidism in patients with Hashimoto's thyroiditis. N. Engl. J. Med. 352, 518–519 (2005).
Novartis Pharmaceuticals Corporation. Gleevec (imatinib mesylate): full prescribing information. Novartis Pharmaceuticals Corporation, East Hanover, NJ (2002).
Wang, J. F. et al. Presence and possible role of vascular endothelial growth factor in thyroid cell growth and function. J. Endocrinol. 157, 5–12 (1998).
Gordon, M. S. et al. Phase I safety and pharmacokinetic study of recombinant human anti-vascular endothelial growth factor in patients with advanced cancer. J. Clin. Oncol. 19, 843–850 (2001).
Willett, C. G. et al. Surrogate markers for antiangiogenic therapy and dose-limiting toxicities for bevacizumab with radiation and chemotherapy: continued experience of a phase I trial in rectal cancer patients. J. Clin. Oncol. 23, 8136–8139 (2005).
Willett, C. G. et al. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat. Med. 10, 145–147 (2004).
Grossmann, M., Premaratne, E., Desai, J. & Davis, I. D. Thyrotoxicosis during sunitinib treatment for renal cell carcinoma. Clin. Endocrinol. (Oxf.) 69, 669–672 (2008).
Mendel, D. B. et al. In vivo antitumour activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
Takahashi, M. et al. Cloning and expression of the ret proto-oncogene encoding a receptor tyrosine kinase with two potential transmembrane domains. Oncogene 3, 571–578 (1988).
Nakamura, T., Ishizaka, Y., Nagao, M., Hara, M. & Ishikawa, T. Expression of the ret proto-oncogene product in human normal and neoplastic tissues of neural crest origin. J. Pathol. 172, 255–260 (1994).
Chiloeches, A. & Marais, R. Is BRAF the Achilles' heel of thyroid cancer? Clin. Cancer Res. 12, 1661–1664 (2006).
Motzer, R. J. et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N. Engl. J. Med. 356, 115–124 (2007).
Escudier, B. et al. Sorafenib in advanced clear-cell renal-cell carcinoma. N. Engl. J. Med. 356, 125–134 (2007).
Ryan, C. W. et al. Sorafenib with interferon alfa-2b as first-line treatment of advanced renal carcinoma: a phase II study of the Southwest Oncology Group. J. Clin. Oncol. 22, 3296–3301 (2007).
Wolter, P., Dumez, H. & Schöffski, P. Laboratory abnormalities suggesting thyroid dysfunction in patients treated with sunitinib. Ann. Intern. Med. [http://www.annals.org/cgi/eletters/145/9/660] (2007).
Garfield, D. H., Hercbergs, A. & Davis, P. J. Re: Hypothyroidism in patients with metastatic renal cell carcinoma treated with sunitinib. J. Natl Cancer Inst. 99, 975–976 (2007).
Davis, P. J. et al. Cell surface receptor for thyroid hormone and tumor cell proliferation. Exp. Rev. Endocrinol. Metabol. 1, 753–761 (2007).
Surks, M. I. et al. Subclinical thyroid disease: scientific review and guidelines for diagnosis and management. JAMA. 291, 228–238 (2004).
Garfield, D., Hercbergs, A. & Davis P. Unanswered questions regarding the management of sunitinib-induced hypothyroidism. Nat. Clin. Pract. Oncol. 4, 674–675 (2007).
Baffert, F. et al. Cellular changes in normal blood capillaries undergoing regression after inhibition of VEGF signaling. Am. J. Physiol. Heart Circ. Physiol. 290, H547–H559 (2006).
Kamba, T. et al. VEGF-dependent plasticity of fenestrated capillaries in the normal adult microvasculature. Am. J. Physiol. Heart Circ. Physiol. 290, H560–H576 (2006).
Maitland, M. L. & Ratain, M. J. Terminal ballistics of kinase inhibitors: there are no magic bullets. Ann. Intern. Med. 145, 702–703 (2006).
Surks, M. I. & Hollowell, J. G. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J. Clin. Endocrinol. Metab. 92, 4575–4582 (2007).
Cho, J. Y., Sagartz, J. E., Capen, C. C., Mazzaferri, E. L. & Jhiang, S. M. Early cellular abnormalities induced by RET/PTC1 oncogene in thyroid-targeted transgenic mice. Oncogene 18, 3659–3665 (1999).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Torino, F., Corsello, S., Longo, R. et al. Hypothyroidism related to tyrosine kinase inhibitors: an emerging toxic effect of targeted therapy. Nat Rev Clin Oncol 6, 219–228 (2009). https://doi.org/10.1038/nrclinonc.2009.4
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2009.4
This article is cited by
-
Adverse effects of tyrosine kinase inhibitors in cancer therapy: pathophysiology, mechanisms and clinical management
Signal Transduction and Targeted Therapy (2023)
-
Ipotiroidismo indotto da sunitinib
L'Endocrinologo (2022)
-
Thyroid autoimmunity and hypothyroidism are associated with deep molecular response in patients with chronic myeloid leukemia on tyrosine kinase inhibitors
Journal of Endocrinological Investigation (2022)
-
Association between the use of allopurinol and risk of increased thyroid-stimulating hormone level
Scientific Reports (2021)
-
Destructive thyroiditis presenting as thyrotoxicosis followed by hypothyroidism during lenvatinib therapy for hepatocellular carcinoma
Clinical Journal of Gastroenterology (2020)