Abstract
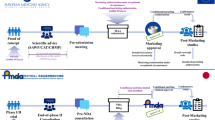
In the past decade, the standards of clinical trials in China have moved closer to international standards, thus encouraging the development of innovative drugs. However, a large backlog of pending applications for both drug approval and clinical trial registration has arisen owing to the complexity of the approval process, the volume of applications and a lack of staff available to process these applications, among other reasons. To improve the drug approval process, a 'four-colour-light' strategy was introduced. Different drugs are classified into redefined categories of innovative and generic drugs, with priority being given to approval decisions concerning innovative drugs. Other improvement strategies are now also being implemented, including the development of a new clinical trial approval system and several measures designed to encourage greater participation of Chinese researchers and research centres in international clinical trials. In this Perspective, the changing landscape of clinical approval in China is described, including the difficulties that drug approval authorities face in this rapidly developing nation and the novel strategies that are being used to find solutions.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout
Similar content being viewed by others
References
National Center for Drug Evaluation (CDE). 2014 Annual Report on Drug Evaluation in China. Yao xue Jin Zhan [Progress In Pharmaceutical Sciences] 39, 241–250 (2015).
China Food and Drug Administration. Guidelines for electronic data collection techniques in clinical trials. sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0087/160963.html (in Chinese) (2016).
China Food and Drug Administration. Administrative measures for drug registration. eng.sfda.gov.cnhttp://eng.sfda.gov.cn/WS03/CL0768/61645.html (2007).
Li, J. et al. Randomized, double-blind, placebo-controlled phase III trial of apatinib in patients with chemotherapy-refractory advanced or metastatic adenocarcinoma of the stomach or gastroesophageal junction. J. Clin. Oncol. 34, 1448–1454 (2016).
Kaufman, H. L., Kohlhapp, F. J. & Zloza, A. Oncolytic viruses: a new class of immunotherapy drugs. Nat. Rev. Drug Discov. 14, 642–662 (2015).
Sheridan, C. First oncolytic virus edges towards approval in surprise vote. Nat. Biotechnol. 33, 569–570 (2015).
Parry, J. Queue for drug registrations grows longer in China. BMJ 350, h1596 (2015).
Wu, Y. L., Zhang, H. & Yang, Y. Cancer drug development in China: recent advances and future challenges. Drug Discov. Today 20, 766–771 (2015).
China Food and Drug Administration. CFDA issued Announcement on Several Policies Pertaining to the Review & Approval of Drug Registration. CCPIEhttp://www.ccpie.org/news/download/2015pharm-13.pdf (2015).
China Food and Drug Administration. Notice of the director-general on the publication of technical guidelines for the management of clinical trial data (No. 112 of 2016). sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0087/160961.html (in Chinese) (2016).
China Food and Drug Administration. Notice of the director-general on guidelines for the planning and reporting of drug administration and statistical analysis of drug clinical trials (No. 113 of 2016). sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0087/160962.html (in Chinese) (2016).
Deng, Y. Z., Wang, H. W. & Fu, H. J. Implementation of performance metrics in clinical trial data management. Yao Xue Xue Bao 50, 1488–1492 (in Chinese) (2015).
Zhang, Z. Big data and clinical research: focusing on the area of critical care medicine in mainland China. Quant. Imaging Med. Surg. 4, 426–429 (2014).
Shi, Y. et al. Icotinib versus gefitinib in previously treated advanced non-small-cell lung cancer (ICOGEN): a randomised, double-blind phase 3 non-inferiority trial. Lancet Oncol. 14, 953–961 (2013).
Shi, Y. et al. Results from a multicenter, open-label, pivotal phase II study of chidamide in relapsed or refractory peripheral T-cell lymphoma. Ann. Oncol. 26, 1766–1771 (2015).
Gavine, P. R. et al. Volitinib, a potent and highly selective c-Met inhibitor, effectively blocks c-Met signaling and growth in c-MET amplified gastric cancer patient-derived tumor xenograft models. Mol. Oncol. 9, 323–333 (2015).
Zhang, Z. K. et al. Icaritin requires phosphatidylinositol 3 kinase (PI3K)/Akt signaling to counteract skeletal muscle atrophy following mechanical unloading. Sci. Rep. 6, 20300 (2016).
Zhao, H. et al. A novel anti-cancer agent Icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget 6, 31927–31943 (2015).
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12, 735–742 (2011).
Wu, Y. L. et al. Erlotinib as second-line treatment in patients with advanced non-small-cell lung cancer and asymptomatic brain metastases: a phase II study (CTONG-0803). Ann. Oncol. 24, 993–999 (2013).
Zhou, Q. et al. Pemetrexed versus gefitinib as a second-line treatment in advanced nonsquamous nonsmall-cell lung cancer patients harboring wild-type EGFR (CTONG0806): a multicenter randomized trial. Ann. Oncol. 25, 2385–2391 (2014).
Zhou, C. et al. Final overall survival results from a randomised, phase III study of erlotinib versus chemotherapy as first-line treatment of EGFR mutation-positive advanced non-small-cell lung cancer (OPTIMAL, CTONG-0802). Ann. Oncol. 26, 1877–1883 (2015).
Simon, R., Geyer, S., Subramanian, J. & Roychowdhury, S. The Bayesian basket design for genomic variant-driven phase II trials. Semin. Oncol. 43, 13–18 (2016).
Menis, J., Hasan, B. & Besse, B. New clinical research strategies in thoracic oncology: clinical trial design, adaptive, basket and umbrella trials, new end-points and new evaluations of response. Eur. Respir. Rev. 23, 367–378 (2014).
Lopez-Chavez, A. et al. Molecular profiling and targeted therapy for advanced thoracic malignancies: a biomarker-derived, multiarm, multihistology phase II basket trial. J. Clin. Oncol. 33, 1000–1007 (2015).
Wildiers, H. et al. End points and trial design in geriatric oncology research: a joint European organisation for research and treatment of cancer — Alliance for Clinical Trials in Oncology — International Society Of Geriatric Oncology position article. J. Clin. Oncol. 31, 3711–3718 (2013).
Zhou, Q. A. Phase II cluster study of single-agent AUY922, BYL719, INC280, LDK378, and MEK162 in Chinese patients with advanced non-small cell lung cancer (NSCLC) [abstract]. J. Clin. Oncol. 32, TPS8122 (2014).
Ke, E. E., Zhou, Q. & Wu, Y. L. Emerging paradigms in targeted treatments for Asian patients with NSCLC. Expert Opin. Pharmacother. 16, 1167–1176 (2015).
US National Library of Medicine. ClinicalTrials.govhttps://clinicaltrials.gov/ct2/show/NCT02276027 (2016).
Li, J., Tian, J., Ma, B. & Yang, K. N-Of-1 trials in China. Complement. Ther. Med. 21, 190–194 (2013).
Kim, E. S. et al. Gefitinib versus docetaxel in previously treated non-small-cell lung cancer (INTEREST): a randomised phase III trial. Lancet 372, 1809–1818 (2008).
Wu, Y. L. et al. Intercalated combination of chemotherapy and erlotinib for patients with advanced stage non-small-cell lung cancer (FASTACT-2): a randomised, double-blind trial. Lancet Oncol. 14, 777–786 (2013).
Ciuleanu, T. et al. Maintenance pemetrexed plus best supportive care versus placebo plus best supportive care for non-small-cell lung cancer: a randomised, double-blind, phase 3 study. Lancet 374, 1432–1440 (2009).
Zhou, C. et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, phase III study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J. Clin. Oncol. 33, 2197–2204 (2015).
Paz-Ares, L. et al. Monotherapy administration of sorafenib in patients with non-small cell lung cancer (MISSION) trial: a phase III, multicenter, placebo-controlled trial of sorafenib in patients with relapsed or refractory predominantly nonsquamous non-small-cell lung cancer after 2 or 3 previous treatment regimens. J. Thorac. Oncol. 10, 1745–1753 (2015).
Lei, Y. Y. et al. Anaplastic lymphoma kinase variants and the percentage of ALK-positive tumor cells and the efficacy of crizotinib in advanced NSCLC. Clin. Lung Cancer 17, 223–231 (2016).
Lei, Y. Y. et al. Clinical efficacy of crizotinib in Chinese patients with ALK-positive non-small-cell lung cancer with brain metastases. J. Thorac. Dis. 7, 1181–1188 (2015).
Solomon, B. J. et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N. Engl. J. Med. 371, 2167–2177 (2014).
Schuler, M. et al. First-line afatinib versus chemotherapy in patients with non-small cell lung cancer and common epidermal growth factor receptor gene mutations and brain metastases. J. Thorac. Oncol. 11, 380–390 (2016).
Wu, Y. L. et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 15, 213–222 (2014).
Yang, J. C. et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 16, 141–151 (2015).
Geater, S. L. et al. Symptom and quality of life improvement in LUX-Lung 6: an open-label phase III study of afatinib versus cisplatin/gemcitabine in Asian patients with EGFR mutation-positive advanced non-small-cell lung cancer. J. Thorac. Oncol. 10, 883–889 (2015).
Reinersman, J. M. et al. Frequency of EGFR and KRAS mutations in lung adenocarcinomas in African Americans. J. Thorac. Oncol. 6, 28–31 (2011).
Wu, Y. L. et al. Epidermal growth factor receptor mutations and their correlation with gefitinib therapy in patients with non-small cell lung cancer: a meta-analysis based on updated individual patient data from six medical centers in mainland China. J. Thorac. Oncol. 2, 430–439 (2007).
Kim, H. J. et al. Clinical investigation of EGFR mutation detection by pyrosequencing in lung cancer patients. Oncol. Lett. 5, 271–276 (2013).
Lynch, T. J. et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 350, 2129–2139 (2004).
Cadranel, J. et al. Impact of systematic EGFR and KRAS mutation evaluation on progression-free survival and overall survival in patients with advanced non-small-cell lung cancer treated by erlotinib in a French prospective cohort (ERMETIC project — part 2). J. Thorac. Oncol. 7, 1490–1502 (2012).
Paez, J. G. et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 304, 1497–1500 (2004).
Dogan, S. et al. Molecular epidemiology of EGFR and KRAS mutations in 3,026 lung adenocarcinomas: higher susceptibility of women to smoking-related KRAS-mutant cancers. Clin. Cancer Res. 18, 6169–6177 (2012).
China Food and Drug Administration. Opinions of the state council on reforming the examination and approval system for drugs and medical devices. sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0056/126821.html (in Chinese) (2015).
China Food and Drug Administration. The national drug review and approval system reform work conference was held in Shanghai. sda.gov.cnhttp://www.cfda.gov.cn/WS01/CL0050/127500.html (in Chinese) (2015).
Lu, X., Ning, Z., Li, Z., Cao, H. & Wang, X. Development of chidamide for peripheral T-cell lymphoma, the first orphan drug approved in China. Intractable Rare Dis. Res. 5, 185–191 (2016).
National Center for Drug Evaluation. Annual drug review report. cde.org.cnhttp://www.cde.org.cn/news.do?method=largeInfo&id=313528 (in Chinese) (2016).
Announcement of the state food and drug administration on carrying out self-checking and checking of drug clinical trial data. sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0087/124800.html (in Chinese) (2016).
Zhu, F. C. et al. Efficacy, safety, and immunology of an inactivated alum-adjuvant enterovirus 71 vaccine in children in China: a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 381, 2024–2032 (2013).
Liao, G. et al. Phase III trial of a Sabin strain-based inactivated poliovirus vaccine. J. Infect. Dis. 214, 1728–1734 (2016).
Zeng, M. et al. Efficacy, safety, and immunogenicity of an oral recombinant Helicobacter pylori vaccine in children in China: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 386, 1457–1464 (2015).
Wu, S. et al. An adenovirus vaccine expressing ebola virus variant makona glycoprotein is efficacious in guinea pigs and nonhuman primates. J. Infect. Dis. 214, S326–S332 (2016).
China Food and Drug Administration. Opinions of the general administration of the people's republic of China on implementing the priority examination and approval of the drug registration application. sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0844/145260.html (in Chinese) (2016).
China Food and Drug Administration. Notice of the general administration of the people's republic of China on releasing the work program for the reform of the classification of chemical drugs registration. sda.gov.cnhttp://www.sda.gov.cn/WS01/CL0087/146140.html (in Chinese) (2016).
[CFDA Wu zhen: 3 years to solve the problem of drug approval]. Zhongguo Yaodian [Chinese pharmacies] (2015).
Huang, J. et al. Icotinib in patients with pretreated advanced esophageal squamous cell carcinoma with EGFR overexpression or EGFR gene amplification: a single-arm, multicenter phase 2 study. J. Thorac. Oncol. 11, 910–917 (2016).
Zhao, Q. et al. Phase I study of icotinib hydrochloride (BPI-2009H), an oral EGFR tyrosine kinase inhibitor, in patients with advanced NSCLC and other solid tumors. Lung Cancer 73, 195–202 (2011).
Li, J. et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J. Clin. Oncol. 31, 3219–3225 (2013).
Tian, S. et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 102, 1374–1380 (2011).
Dong, M. et al. Phase I study of chidamide (CS055/HBI-8000), a new histone deacetylase inhibitor, in patients with advanced solid tumors and lymphomas. Cancer Chemother. Pharmacol. 69, 1413–1422 (2012).
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation and reviewing/editing of this manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Zhou, Q., Chen, XY., Yang, ZM. et al. The changing landscape of clinical trial and approval processes in China. Nat Rev Clin Oncol 14, 577–583 (2017). https://doi.org/10.1038/nrclinonc.2017.10
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2017.10
This article is cited by
-
Analysis of Incentive Policies and Initiatives on Orphan Drug Development in China: Challenges, Reforms and Implications
Orphanet Journal of Rare Diseases (2023)
-
The changing landscape of drug clinical trials on cardiometabolic diseases in China, 2009–2021
Diabetology & Metabolic Syndrome (2023)
-
Characteristics and Trends in Clinical Trials of Cardiovascular Drugs in China from 2009 to 2021
American Journal of Cardiovascular Drugs (2023)
-
Trends and Characteristics of New Drug Approvals in China, 2011–2021
Therapeutic Innovation & Regulatory Science (2023)
-
Awareness of Clinical Research Coordinators Toward Ethics and Protection of Clinical Trial Patients
Therapeutic Innovation & Regulatory Science (2023)