Abstract
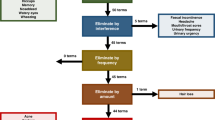
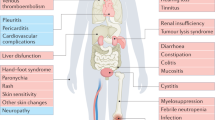
Symptomatic toxicities associated with anticancer treatments, such as nausea and vomiting, are frequently underreported by clinicians, even when data are prospectively collected within clinical trials. Such underreporting can result in an underestimation of the absolute rate of toxicity, which is highly relevant information for patients and their physicians in clinical practice, and for regulatory authorities. Systematic collection of patient-reported outcomes (PROs) has been demonstrated to be a valid, reliable, feasible and precise approach to tabulating symptomatic toxicities and enables symptoms that are missed by clinicians to be detected. In this Perspectives, the barriers and challenges that should be addressed when considering broad integration of PRO toxicity monitoring in oncology clinical trials are discussed, including challenges related to data collection logistics, analytical approaches, and resource utilization. Instruments conceived to enable description of treatment-related adverse effects, from the patient perspective, bring the potential to improve risk-versus-benefit analyses in clinical research, and to provide patients with accurate information, on the basis of previous experiences of their peers.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
Change history
27 January 2016
In the original version of this article published online, the title and doi of reference 43 was incorrect. These errors have been corrected in the online and print versions of the article.
References
U.S. Food and Drug administration. Food and Drug Administration's overall risk management activities. [online], (2015).
European Medicines Agency. Reflection paper on benefit-risk assessment methods in the context of the evaluation of marketing authorisation application of medicinal products for human use. [online], (2008).
European Medicines Agency. Guideline on the evaluation of anticancer products in man. [online], (2012).
The National Cancer Institute. Common terminology criteria for adverse events, version 3.0. [online], (2006).
U.S. Food and Drug Administration. Clinical Outcome Assessment (COA): glossary of terms. [online], (2015).
Atkinson, T. M. et al. Reliability of adverse symptom event reporting by clinicians. Qual. Life Res. 21, 1159–1164 (2012).
Basch, E. The missing voice of patients in drug-safety reporting. N. Engl. J. Med. 362, 865–869 (2010).
Basch, E. et al. Adverse symptom event reporting by patients versus clinicians: relationships with clinical outcomes. J. Natl Cancer Inst. 101, 1624–1632 (2009).
Basch, E. et al. Development of the National Cancer Institute's patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE). J. Natl Canc. Inst. 106, 9 (2014).
U.S. Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development to support labeling claims. [online], (2015).
Trotti, A., Colevas, A. D., Setser, A. & Basch, E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J. Clin. Oncol. 25, 5121–5127 (2007).
Reeve, B. B. et al. Recommended patient-reported core set of symptoms to measure in adult cancer treatment trials. J. Natl Cancer Inst. 106, 7 (2014).
European Medicines Agency. Reflection paper on the regulatory guidance for the use of health-related quality of life (HRQL) measures in the evaluation of medicinal products. [online], (2005).
European Medicines Agency. Reflection paper on the use of patient reported outcome (PRO) measures in oncology studies. [online], (2014).
Ruddy, K., Mayer, E. & Partridge, A. Patient adherence and persistence with oral anticancer treatment. CA Cancer J. Clin. 59, 56–66 (2009).
Hadji, P. et al. COMPliance and Arthralgia in Clinical Therapy: the COMPACT trial, assessing the incidence of arthralgia, and compliance within the first year of adjuvant anastrozole therapy. Ann. Oncol. 25, 372–377 (2014).
Naidoo, J. et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies. Ann. Oncol. 26, 2375–2391 (2015).
Grossman, S. A., Sheidler, V. R., Swedeen, K., Mucenski, J. & Piantadosi, S. Correlation of patient and caregiver ratings of cancer pain. J. Pain Symptom Manage. 6, 53–57 (1991).
Vogelzang, N. J. et al. Patient, caregiver, and oncologist perceptions of cancer-related fatigue: results of a tripart assessment survey. The Fatigue Coalition. Semin. Hematol. 34, S4–S12 (1997).
Litwin, M. S., Lubeck, D. P., Henning, J. M. & Carroll, P. R. Differences in urologist and patient assessments of health related quality of life in men with prostate cancer: results of the CaPSURE database. J. Urol. 159, 1988–1992 (1998).
Petersen, M. A., Larsen, H., Pedersen, L., Sonne, N. & Groenvold, M. Assessing health-related quality of life in palliative care: comparing patient and physician assessments. Eur. J. Cancer 42, 1159–1166 (2006).
Fromme, E. K. Eilers, K. M., Mori, M., Hsieh, Y. C. & Beer, T. M. How accurate is clinician reporting of chemotherapy adverse effects? A comparison with patient-reported symptoms from the Quality-of-Life Questionnaire C30. J. Clin. Oncol. 22, 3485–3490 (2004).
Basch, E. et al. Patient versus clinician symptom reporting using the National Cancer Institute Common Terminology Criteria for Adverse Events: results of a questionnaire-based study. Lancet Oncol. 7, 903–909 (2006).
Cirillo, M. et al. Clinician versus nurse symptom reporting using the National Cancer Institute-Common Terminology Criteria for Adverse Events during chemotherapy: results of a comparison based on patient's self-reported questionnaire. Ann. Oncol. 20, 1929–1935 (2009).
Di Maio, M. et al. Symptomatic toxicities experienced during anticancer treatment: agreement between patient and physician reporting in three randomized trials. J. Clin. Oncol. 33, 910–915 (2015).
Wu, A. W., Snyder, C., Clancy, C. M. & Steinwachs, D. M. Adding the patient perspective to comparative effectiveness research. Health Aff. (Millwood) 29, 1863–1871 (2010).
Dueck, A. et al. A cluster-randomized study of clinician-patient shared versus standard reporting of symptomatic adverse events using PRO-CTCAE nested in a multicenter trial of multimodal therapy for rectal cancer (Alliance N1048 PROSPECT). [abstract], Qual. Life Res. 24, S1–S2 (2015).
Basch, E. et al. Feasibility of patient reporting of symptomatic adverse events via the PRO-CTCAE in a Radiation Therapy Oncology Group (RTOG) Cooperative Group Clinical Trial. [abstract], Qual. Life Res. 23, (Suppl. 1) 97–98 (2014).
Basch, E. et al. Recommendations for incorporating patient-reported outcomes into clinical comparative-effectiveness research in adult oncology. J. Clin. Oncol. 30, 4249–4255 (2012).
Bruner, D. W. et al. Stakeholder perspectives on implementing the National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Transl. Behav. Med. 1, 110–122 (2011).
Verstovsek, S. et al. A double-blind, placebo-controlled trial of ruxolitinib for myelofibrosis. N. Engl. J. Med. 366, 799–807 (2012).
Stull, D. E. & Leidy, N. K. Parasuraman, B. & Chassany, O. Optimal recall periods for patient-reported outcomes: challenges and potential solutions. Curr. Med. Res. Opin. 25, 929–942 (2009).
Mendoza, T. R. et al. Impact of recall period on the accuracy of selected items from the US National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). [abstract], Qual. Life Res. 23, (Suppl. 1) 141–142 (2014).
Basch, E. et al. Feasibility and clinical impact of sharing patient-reported symptom toxicities and performance status with clinical investigators during a phase 2 cancer treatment trial. Clin. Trials http://dx.doi.org/10.1177/1740774515615540 (2015).
Hay, J. L. et al. Cognitive interviewing of the U.S. National Cancer Institute's Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). Qual. Life Res. 23, 257–269 (2014).
Dueck, A. C. et al. Validity and reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol. 1, 1051–1059 (2015).
Basch, E., Bennett, A. & Pietanza, M. C. Use of patient-reported outcomes to improve the predictive accuracy of clinician-reported adverse events. J. Natl Cancer Inst. 103, 1808–1810 (2011).
Movsas, B. PROceeding with the Patient-Reported Outcomes (PROs) Version of the Common Terminology Criteria for Adverse Events. JAMA Oncol. 1, 1059–1060 (2015).
U.S. National Institutes of Health. Healthcare delivery research program. [online], (2015).
Fellowes, D., Fallowfield, L. J., Saunders, C. M. & Houghton, J. Tolerability of hormone therapies for breast cancer: how informative are documented symptom profiles in medical notes for 'well-tolerated' treatments? Breast Cancer Res. Treat. 66, 73–81 (2001).
Novello, S. et al. Italian multicenter survey to evaluate the opinion of patients and their reference clinicians on the 'tolerance' to targeted therapies already available for non-small cell lung cancer treatment in daily clinical practice. Transl. Lung Cancer Res. 3, 173–180 (2014).
Gravis, G. et al. Patients' self-assessment versus investigators' evaluation in a phase III trial in non-castrate metastatic prostate cancer (GETUG-AFU 15). Eur. J. Cancer 50, 953–962 (2014).
Montemurro, F. et al. Self-evaluation of adjuvant chemotherapy-related adverse effects by patients with breast cancer. JAMA Oncol. http://dx.doi.org/10.1001/jamaoncol.2015.4720 (2015).
Acknowledgements
M.D.M. has received research funding from the CRT Foundation (Torino, Italy) for a research project studying the impact on the quality of life of cancer patients of a systematic collection of treatment toxicities by dedicated questionnaires.
Author information
Authors and Affiliations
Contributions
All authors researched data for this article, made a substantial contribution to discussions of content, wrote the manuscript and reviewed and/or edited the manuscript prior to submission.
Corresponding author
Ethics declarations
Competing interests
E.B. has received research funding from the US National Cancer Institute for developing the PRO–CTCAE. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Di Maio, M., Basch, E., Bryce, J. et al. Patient-reported outcomes in the evaluation of toxicity of anticancer treatments. Nat Rev Clin Oncol 13, 319–325 (2016). https://doi.org/10.1038/nrclinonc.2015.222
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.222
This article is cited by
-
Patient-reported outcome (PRO) instruments used in patients undergoing adoptive cell therapy (ACT) for the treatment of cancer: a systematic review
Systematic Reviews (2023)
-
In situ self-assembly for cancer therapy and imaging
Nature Reviews Materials (2023)
-
Patient-physician agreement on function and pain is associated with long-term outcomes in sarcoma: findings from a longitudinal study
Journal of Cancer Survivorship (2023)
-
Willingness to report treatment-related symptoms of immunotherapy among patients with non-small cell lung cancer
Quality of Life Research (2022)
-
Co-occurrence and metabolic biomarkers of sensory and motor subtypes of peripheral neuropathy from paclitaxel
Breast Cancer Research and Treatment (2022)