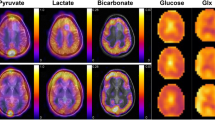
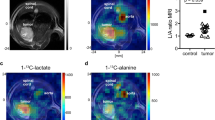
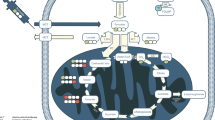
Key Points
-
Brain tumours, such as gliomas, in adults and children are morphologically similar, but harbour different genomic alterations, both across and within histological subtypes, that affect prognosis and treatment response
-
Many of these genomic alterations lead to reprogramming of cellular metabolism, including glucose, amino acid, and lipid metabolism
-
The metabolic reprogramming in brain tumours can be visualized and assessed using various non-invasive clinical imaging modalities
-
Metabolic imaging shows great promise as a means to non-invasively evaluate some of the genomic alterations in order to guide the clinical management of patients
Abstract
The revolution in cancer genomics has uncovered a variety of clinically relevant mutations in primary brain tumours, creating an urgent need to develop non-invasive imaging biomarkers to assess and integrate this genetic information into the clinical management of patients. Metabolic reprogramming is a central hallmark of cancer, including brain tumours; indeed, many of the molecular pathways implicated in the pathogenesis of brain tumours result in reprogramming of metabolism. This relationship provides the opportunity to devise in vivo metabolic imaging modalities to improve diagnosis, patient stratification, and monitoring of treatment response. Metabolic phenomena, such as the Warburg effect and altered mitochondrial metabolism, can be leveraged to image brain tumours using techniques including PET and MRI. Moreover, genetic alterations, such as mutations affecting isocitrate dehydrogenase, are associated with unique metabolic signatures that can be detected using magnetic resonance spectroscopy. The need to translate our understanding of the molecular features of brain tumours into imaging modalities with clinical utility is growing; metabolic imaging provides a unique platform to achieve this objective. In this Review, we examine the molecular basis for metabolic reprogramming in brain tumours, and examine current non-invasive metabolic imaging strategies that can be used to interrogate these molecular characteristics with the ultimate goal of guiding and improving patient care.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Louis, D. N. et al. World Health Organization Classification of Tumors of the Central Nervous Systemrevised 4th edn (International Agency for Research on Cancer (IARC), 2016).
Peng, B. H. & Levin, C. S. Recent development in PET instrumentation. Curr. Pharm. Biotechnol. 11, 555–571 (2010).
Martinez-Outschoorn, U. E., Peiris-Pages, M., Pestell, R. G., Sotgia, F. & Lisanti, M. P. Cancer metabolism: a therapeutic perspective. Nat. Rev. Clin. Oncol. http://dx.doi.org/10.1038/nrclinonc.2016.60 (2016).
Glunde, K. & Bhujwalla, Z. M. Metabolic tumor imaging using magnetic resonance spectroscopy. Semin. Oncol. 38, 26–41 (2011).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Ward, P. S. & Thompson, C. B. Metabolic reprogramming: a cancer hallmark even Warburg did not anticipate. Cancer Cell 21, 297–308 (2012).
Brennan, C. W. et al. The somatic genomic landscape of glioblastoma. Cell 155, 462–477 (2013).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 455, 1061–1068 (2008).
Schwartzentruber, J. et al. Driver mutations in histone H3.3 and chromatin remodelling genes in paediatric glioblastoma. Nature 482, 226–231 (2012).
Wu, G. et al. Somatic histone H3 alterations in pediatric diffuse intrinsic pontine gliomas and non-brainstem glioblastomas. Nat. Genet. 44, 251–253 (2012).
Sturm, D. et al. Hotspot mutations in H3F3A and IDH1 define distinct epigenetic and biological subgroups of glioblastoma. Cancer Cell 22, 425–437 (2012).
Khuong-Quang, D. A. et al. K27M mutation in histone H3.3 defines clinically and biologically distinct subgroups of pediatric diffuse intrinsic pontine gliomas. Acta Neuropathol. 124, 439–447 (2012).
Yan, H. et al. IDH1 and IDH2 mutations in gliomas. N. Engl. J. Med. 360, 765–773 (2009).
Venneti, S. & Huse, J. T. The evolving molecular genetics of low-grade glioma. Adv. Anat. Pathol. 22, 94–101 (2015).
Remke, M., Ramaswamy, V. & Taylor, M. D. Medulloblastoma molecular dissection: the way toward targeted therapy. Curr. Opin. Oncol. 25, 674–681 (2013).
Gajjar, A. J. & Robinson, G. W. Medulloblastoma-translating discoveries from the bench to the bedside. Nat. Rev. Clin. Oncol. 11, 714–722 (2014).
Robinson, G. W. et al. Vismodegib exerts targeted efficacy against recurrent Sonic Hedgehog-subgroup medulloblastoma: results from phase II Pediatric Brain Tumor Consortium Studies PBTC-025B and PBTC-032. J. Clin. Oncol. 33, 2646–2654 (2015).
Robinson, G. W., Orr, B. A. & Gajjar, A. Complete clinical regression of a BRAF V600E-mutant pediatric glioblastoma multiforme after BRAF inhibitor therapy. BMC Cancer 14, 258 (2014).
Bautista, F. et al. Vemurafenib in pediatric patients with BRAFV600E mutated high-grade gliomas. Pediatr. Blood Cancer 61, 1101–1103 (2014).
Rush, S., Foreman, N. & Liu, A. Brainstem ganglioglioma successfully treated with vemurafenib. J. Clin. Oncol. 31, e159–e160 (2013).
Rohle, D. et al. An inhibitor of mutant IDH1 delays growth and promotes differentiation of glioma cells. Science 340, 626–630 (2013).
Maher, E. A. et al. Metabolism of [U-13C]glucose in human brain tumors in vivo. NMR Biomed. 25, 1234–1244 (2012).
Lunt, S. Y. & Vander Heiden, M. G. Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 (2011).
Baumann, F. et al. Lactate promotes glioma migration by TGF-β2-dependent regulation of matrix metalloproteinase-2. Neuro Oncol. 11, 368–380 (2009).
Colen, C. B. et al. Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia 13, 620–632 (2011).
Crane, C. A. et al. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc. Natl Acad. Sci. USA 111, 12823–12828 (2014).
Kohn, A. D., Summers, S. A., Birnbaum, M. J. & Roth, R. A. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J. Biol. Chem. 271, 31372–31378 (1996).
Deprez, J., Vertommen, D., Alessi, D. R., Hue, L. & Rider, M. H. Phosphorylation and activation of heart 6-phosphofructo-2-kinase by protein kinase B and other protein kinases of the insulin signaling cascades. J. Biol. Chem. 272, 17269–17275 (1997).
Gottlob, K. et al. Inhibition of early apoptotic events by Akt/PKB is dependent on the first committed step of glycolysis and mitochondrial hexokinase. Genes Dev. 15, 1406–1418 (2001).
Babic, I. et al. EGFR mutation-induced alternative splicing of Max contributes to growth of glycolytic tumors in brain cancer. Cell Metab. 17, 1000–1008 (2013).
Osthus, R. C. et al. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 275, 21797–21800 (2000).
Hu, S. et al. 13C-pyruvate imaging reveals alterations in glycolysis that precede c-Myc-induced tumor formation and regression. Cell Metab. 14, 131–142 (2011).
Parmenter, T. J. et al. Response of BRAF-mutant melanoma to BRAF inhibition is mediated by a network of transcriptional regulators of glycolysis. Cancer Discov. 4, 423–433 (2014).
Kruiswijk, F., Labuschagne, C. F. & Vousden, K. H. p53 in survival, death and metabolic health: a lifeguard with a licence to kill. Nat. Rev. Mol. Cell Biol. 16, 393–405 (2015).
Wolf, A., Agnihotri, S., Munoz, D. & Guha, A. Developmental profile and regulation of the glycolytic enzyme hexokinase 2 in normal brain and glioblastoma multiforme. Neurobiol. Dis. 44, 84–91 (2011).
Wolf, A. et al. Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 208, 313–326 (2011).
Gershon, T. R. et al. Hexokinase-2-mediated aerobic glycolysis is integral to cerebellar neurogenesis and pathogenesis of medulloblastoma. Cancer Metab. 1, 2 (2013).
Patra, K. C. et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228 (2013).
Di Magno, L. et al. Druggable glycolytic requirement for Hedgehog-dependent neuronal and medulloblastoma growth. Cell Cycle 13, 3404–3413 (2014).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Kefas, B. et al. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro Oncol. 12, 1102–1112 (2010).
Mukherjee, J. et al. Pyruvate kinase M2 expression, but not pyruvate kinase activity, is up-regulated in a grade-specific manner in human glioma. PLoS ONE 8. e57610 (2013).
Witney, T. H. et al. PET imaging of tumor glycolysis downstream of hexokinase through noninvasive measurement of pyruvate kinase M2. Sci. Transl. Med. 7, 310ra169 (2015).
Kelloff, G. J. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin. Cancer Res. 11, 2785–2808 (2005).
Patronas, N. J. et al. Prediction of survival in glioma patients by means of positron emission tomography. J. Neurosurg. 62, 816–822 (1985).
Colavolpe, C. et al. Independent prognostic value of pre-treatment 18-FDG-PET in high-grade gliomas. J. Neurooncol 107, 527–535 (2011).
Omuro, A. M. P., Leite, C. C., Mokhtari, K. & Delattre, J.-Y. Pitfalls in the diagnosis of brain tumours. Lancet Neurol. 5, 937–948 (2006).
Marin-Valencia, I. et al. Analysis of tumor metabolism reveals mitochondrial glucose oxidation in genetically diverse human glioblastomas in the mouse brain in vivo. Cell Metab. 15, 827–837 (2012).
Kurhanewicz, J., Bok, R., Nelson, S. J. & Vigneron, D. B. Current and potential applications of clinical 13C MR spectroscopy. J. Nucl. Med. 49, 341–344 (2008).
Ardenkjaer-Larsen, J. H. et al. Increase in signal-to-noise ratio of >10,000 times in liquid-state NMR. Proc. Natl Acad. Sci. USA 100, 10158–10163 (2003).
Park, I. et al. Hyperpolarized 13C magnetic resonance metabolic imaging: application to brain tumors. Neuro Oncol. 12, 133–144 (2010).
van Zijl, P. C. & Yadav, N. N. Chemical exchange saturation transfer (CEST): what is in a name and what isn't? Magn. Reson. Med. 65, 927–948 (2011).
Gizewski, E. R., Monninghoff, C. & Forsting, M. Perspectives of ultra-high-field MRI in neuroradiology. Clin. Neuroradiol. 25 (Suppl. 2), 267–273 (2015).
Shah, N. J. Multimodal neuroimaging in humans at 9.4 T: a technological breakthrough towards an advanced metabolic imaging scanner. Brain Struct. Funct. 220, 1867–1884 (2015).
Cai, K. et al. The impact of gabapentin administration on brain GABA and glutamate concentrations: a 7T 1H-MRS study. Neuropsychopharmacology 37, 2764–2771 (2012).
Ren, J., Sherry, A. D. & Malloy, C. R. A simple approach to evaluate the kinetic rate constant for ATP synthesis in resting human skeletal muscle at 7 T. NMR Biomed. http://dx.doi.org/10.1002/nbm.3310 (2015).
Duyn, J. H. The future of ultra-high field MRI and fMRI for study of the human brain. NeuroImage 62, 1241–1248 (2012).
Walker-Samuel, S. et al. In vivo imaging of glucose uptake and metabolism in tumors. Nat. Med. 19, 1067–1072 (2013).
Sagiyama, K. et al. In vivo chemical exchange saturation transfer imaging allows early detection of a therapeutic response in glioblastoma. Proc. Natl Acad. Sci. USA 111, 4542–4547 (2014).
Puttick, S., Bell, C., Dowson, N., Rose, S. & Fay, M. PET, MRI, and simultaneous PET/MRI in the development of diagnostic and therapeutic strategies for glioma. Drug Discov. Today 20, 306–317 (2015).
Dang, L. et al. Cancer-associated IDH1 mutations produce 2-hydroxyglutarate. Nature 465, 966 (2010).
Ward, P. S. et al. The common feature of leukemia-associated IDH1 and IDH2 mutations is a neomorphic enzyme activity converting alpha-ketoglutarate to 2-hydroxyglutarate. Cancer Cell 17, 225–234 (2010).
Koivunen, P. et al. Transformation by the (R)-enantiomer of 2-hydroxyglutarate linked to EGLN activation. Nature 483, 484–488 (2012).
Losman, J. A. et al. (R)-2-hydroxyglutarate is sufficient to promote leukemogenesis and its effects are reversible. Science 339, 1621–1625 (2013).
Noushmehr, H. et al. Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 17, 510–522 (2010).
Lu, C. et al. IDH mutation impairs histone demethylation and results in a block to cell differentiation. Nature 483, 474–478 (2012).
Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483, 479–483 (2012).
Venneti, S. et al. in American Association of Neuorpathologists (Journal of Neuropathology & Experimental Neurology, 2012).
Choi, C. et al. 2-Hydroxyglutarate detection by magnetic resonance spectroscopy in IDH-mutated patients with gliomas. Nat. Med. 18, 624–629 (2012).
Andronesi, O. C. et al. Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy. Sci. Transl. Med. 4, 116ra4 (2012).
Emir, U. E. et al. Noninvasive quantification of 2-hydroxyglutarate in human gliomas with IDH1 and IDH2 mutations. Cancer Res. 76, 43–49 (2016).
Bertolino, N. et al. Accuracy of 2-hydroxyglutarate quantification by short-echo proton-MRS at 3 T: a phantom study. Phys. Med. 30, 702–707 (2014).
Natsumeda, M. et al. Accumulation of 2-hydroxyglutarate in gliomas correlates with survival: a study by 3.0-tesla magnetic resonance spectroscopy. Acta Neuropathol. Commun. 2, 158 (2014).
Hartmann, C. et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 120, 707–718 (2010).
de la Fuente, M. I. et al. Integration of 2-hydroxyglutarate-proton magnetic resonance spectroscopy into clinical practice for disease monitoring in isocitrate dehydrogenase-mutant glioma. Neuro Oncol. 18, 283–290 (2016).
Chaumeil, M. M. et al. Non-invasive in vivo assessment of IDH1 mutational status in glioma. Nat. Commun. 4, 2429 (2013).
Chawla, S. et al. Role of proton magnetic resonance spectroscopy in differentiating oligodendrogliomas from astrocytomas. J. Neuroimaging 20, 3–8 (2010).
Chawla, S. et al. Arterial spin-labeling and MR spectroscopy in the differentiation of gliomas. AJNR Am. J. Neuroradiol. 28, 1683–1689 (2007).
Majos, C. et al. Brain tumor classification by proton MR spectroscopy: comparison of diagnostic accuracy at short and long TE. AJNR Am. J. Neuroradiol. 25, 1696–1704 (2004).
Wilson, M. et al. Noninvasive detection of glutamate predicts survival in pediatric medulloblastoma. Clin. Cancer Res. 20, 4532–4539 (2014).
Koglin, N. et al. Specific PET imaging of xC- transporter activity using a 18F-labeled glutamate derivative reveals a dominant pathway in tumor metabolism. Clin. Cancer Res. 17, 6000–6011 (2011).
Baek, S. et al. Exploratory clinical trial of (4S)-4- (3-[18F]fluoropropyl)-l-glutamate for imaging xC− transporter using positron emission tomography in patients with non-small cell lung or breast cancer. Clin. Cancer Res. 18, 5427–5437 (2012).
Takeuchi, S. et al. Increased xCT expression correlates with tumor invasion and outcome in patients with glioblastomas. Neurosurgery 72, 33–41; discussion 41 (2013).
Sleire, L. et al. Drug repurposing: sulfasalazine sensitizes gliomas to gamma knife radiosurgery by blocking cystine uptake through system Xc−, leading to glutathione depletion. Oncogene 34, 5951–5959 (2015).
DeBerardinis, R. J. et al. Beyond aerobic glycolysis: transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl Acad. Sci. USA 104, 19345–19350 (2007).
Venneti, S. et al. Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo. Sci. Transl. Med. 7, 274ra17 (2015).
Wise, D. R. & Thompson, C. B. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 (2010).
Daye, D. & Wellen, K. E. Metabolic reprogramming in cancer: unraveling the role of glutamine in tumorigenesis. Semin. Cell Dev. Biol. 23, 362–369 (2012).
Wise, D. R. et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl Acad. Sci. USA 105, 18782–18787 (2008).
Gao, P. et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009).
Yuneva, M. O. et al. The metabolic profile of tumors depends on both the responsible genetic lesion and tissue type. Cell Metab. 15, 157–170 (2012).
Qing, G. et al. ATF4 regulates MYC-mediated neuroblastoma cell death upon glutamine deprivation. Cancer Cell 22, 631–644 (2012).
Csibi, A. et al. The mTORC1 pathway stimulates glutamine metabolism and cell proliferation by repressing SIRT4. Cell 153, 840–854 (2013).
Zhang, C. et al. Glutaminase 2 is a novel negative regulator of small GTPase Rac1 and mediates p53 function in suppressing metastasis. eLife 5, e10727 (2016).
Tardito, S. et al. Glutamine synthetase activity fuels nucleotide biosynthesis and supports growth of glutamine-restricted glioblastoma. Nat. Cell Biol. 17, 1556–1568 (2015).
Muzi, M. et al. Kinetic analysis of 3′-deoxy-3′-18F- fluorothymidine in patients with gliomas. J. Nucl. Med. 47, 1612–1621 (2006).
Gulyas, B., Nyary, I. & Borbely, K. F.D. G. MET or CHO? The quest for the optimal PET tracer for glioma imaging continues. Nat. Clin. Pract. Neurol. 4, 470–471 (2008).
Locasale, J. W. Serine, glycine and one-carbon units: cancer metabolism in full circle. Nat. Rev. Cancer 13, 572–583 (2013).
Yun, J., Johnson, J. L., Hanigan, C. L. & Locasale, J. W. Interactions between epigenetics and metabolism in cancers. Front. Oncol. 2, 163 (2012).
Roelcke, U. et al. Alteration of blood–brain barrier in human brain tumors: comparison of [18F]fluorodeoxyglucose, [11C]methionine and rubidium-82 using PET. J. Neurol. Sci. 132, 20–27 (1995).
Sasajima, T. et al. Proliferation-dependent changes in amino acid transport and glucose metabolism in glioma cell lines. Eur. J. Nuclear Med. Mol. Imaging 31, 1244–1256 (2004).
Glaudemans, A. W. J. M. et al. Value of 11C-methionine PET in imaging brain tumours and metastases. Eur. J. Nuclear Med. Mol. Imaging 40, 615–635 (2012).
Ariyannur, P. S., Madhavarao, C. N. & Namboodiri, A. M. N-acetylaspartate synthesis in the brain: mitochondria versus microsomes. Brain Res. 1227, 34–41 (2008).
Rigotti, D. J., Inglese, M. & Gonen, O. Whole-brain N-acetylaspartate as a surrogate marker of neuronal damage in diffuse neurologic disorders. AJNR Am. J. Neuroradiol. 28, 1843–1849 (2007).
Baslow, M. H. N-acetylaspartate in the vertebrate brain: metabolism and function. Neurochem. Res. 28, 941–953 (2003).
Goldstein, F. B. Biosynthesis of N-acetyl-l-aspartic acid. Biochim. Biophys. Acta 33, 583–584 (1959).
Moffett, J., Ross, B., Arun, P., Madhavarao, C. & Namboodiri, A. N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog. Neurobiol. 81, 89–131 (2007).
Juhasz, C., Dwivedi, S., Kamson, D. O., Michelhaugh, S. K. & Mittal, S. Comparison of amino acid positron emission tomographic radiotracers for molecular imaging of primary and metastatic brain tumors. Mol. Imaging http://dx.doi.org/10.2310/7290.2014.00015 (2014).
Dunet, V., Rossier, C., Buck, A., Stupp, R. & Prior, J. O. Performance of 18F-fluoro-ethyl-tyrosine (18F-FET) PET for the differential diagnosis of primary brain tumor: a systematic review and metaanalysis. J. Nucl. Med. 53, 207–214 (2012).
Jansen, N. L. et al. Prediction of oligodendroglial histology and LOH 1p/19q using dynamic [18F]FET-PET imaging in intracranial WHO grade II and III gliomas. Neuro Oncol. 14, 1473–1480 (2012).
Thon, N. et al. Dynamic 18F-FET PET in suspected WHO grade II gliomas defines distinct biological subgroups with different clinical courses. Int. J. Cancer 136, 2132–2145 (2015).
Dolma, S. et al. Inhibition of dopamine receptor D4 impedes autophagic flux, proliferation, and survival of glioblastoma stem cells. Cancer Cell 29, 859–873 (2016).
Ishiwata, K. et al. Re-evaluation of amino acid PET studies: can the protein synthesis rates in brain and tumor tissues be measured in vivo? J. Nucl. Med. 34, 1936–1943 (1993).
Kinoshita, Y. & Yokota, A. Absolute concentrations of metabolites in human brain tumors using in vitro proton magnetic resonance spectroscopy. NMR Biomed. 10, 2–12 (1997).
Nelson, S. J. et al. In vivo molecular imaging for planning radiation therapy of gliomas: an application of 1H MRSI. J. Magn. Reson. Imaging 16, 464–476 (2002).
Chernov, M. F. et al. Multivoxel proton MRS for differentiation of radiation-induced necrosis and tumor recurrence after gamma knife radiosurgery for brain metastases. Brain Tumor Pathol. 23, 19–27 (2006).
Guo, D. et al. EGFR signaling through an Akt-SREBP-1-dependent, rapamycin-resistant pathway sensitizes glioblastomas to antilipogenic therapy. Sci. Signal. 2, ra82 (2009).
Lee, J. V. et al. Akt-dependent metabolic reprogramming regulates tumor cell histone acetylation. Cell Metab. 20, 306–319 (2014).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2012).
Mullen, A. R. et al. Reductive carboxylation supports growth in tumour cells with defective mitochondria. Nature 481, 385–388 (2012).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Yamamoto, Y. et al. 11C-acetate PET in the evaluation of brain glioma: comparison with 11C-methionine and 18F-FDG-PET. Mol. Imaging Biol. 10, 281–287 (2008).
Yamane, T., Sakamoto, S. & Senda, M. Clinical impact of 11C-methionine PET on expected management of patients with brain neoplasm. Eur. J. Nucl. Med. Mol. Imaging 37, 685–690 (2010).
Shields, A. F. et al. Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat. Med. 4, 1334–1336 (1998).
Bading, J. R. & Shields, A. F. Imaging of cell proliferation: status and prospects. J. Nucl. Med. 49 (Suppl. 2), 64S–80S (2008).
McKinley, E. T. et al. Limits of [18F]-FLT PET as a biomarker of proliferation in oncology. PLoS ONE 8, e58938 (2013).
Chen, W. et al. Imaging proliferation in brain tumors with 18F-FLT PET: comparison with 18F-FDG. J. Nucl. Med. 46, 945–952 (2005).
Jacobs, A. H. et al. 18F-fluoro-l-thymidine and 11C-methylmethionine as markers of increased transport and proliferation in brain tumors. J. Nucl. Med. 46, 1948–1958 (2005).
Ackerman, D. & Simon, M. C. Hypoxia, lipids, and cancer: surviving the harsh tumor microenvironment. Trends Cell Biol. 24, 472–478 (2014).
Lee, S. T. & Scott, A. M. Hypoxia positron emission tomography imaging with 18F-fluoromisonidazole. Semin. Nucl. Med. 37, 451–461 (2007).
Bruehlmeier, M., Roelcke, U., Schubiger, P. A. & Ametamey, S. M. Assessment of hypoxia and perfusion in human brain tumors using PET with 18F-fluoromisonidazole and 15O-H2O. J. Nucl. Med. 45, 1851–1859 (2004).
Jain, R. K. et al. Angiogenesis in brain tumours. Nat. Rev. Neurosci. 8, 610–622 (2007).
Haubner, R., Beer, A. J., Wang, H. & Chen, X. Positron emission tomography tracers for imaging angiogenesis. Eur. J. Nucl. Med. Mol. Imaging 37, S86–S103 (2010).
Schnell, O. et al. Imaging of integrin αvβ3 expression in patients with malignant glioma by [18F] Galacto-RGD positron emission tomography. Neuro Oncol. 11, 861–870 (2009).
Tsien, C. I. et al. Concurrent temozolomide and dose-escalated intensity-modulated radiation therapy in newly diagnosed glioblastoma. Clin. Cancer Res. 18, 273–279 (2011).
Kunz, M. et al. Hot spots in dynamic 18FET-PET delineate malignant tumor parts within suspected WHO grade II gliomas. Neuro Oncol. 13, 307–316 (2011).
Arbizu, J. et al. Quantitative volumetric analysis of gliomas with sequential MRI and 11C-methionine PET assessment: patterns of integration in therapy planning. Eur. J. Nucl. Med. Mol. Imaging 39, 771–781 (2012).
Pirotte, B. et al. PET in stereotactic conditions increases the diagnostic yield of brain biopsy. Stereotact. Funct. Neurosurg. 63, 144–149 (1994).
Tanaka, Y. et al. Glioma surgery using a multimodal navigation system with integrated metabolic images. J. Neurosurg. 110, 163–172 (2009).
Di Costanzo, A. et al. Multiparametric 3T MR approach to the assessment of cerebral gliomas: tumor extent and malignancy. Neuroradiology 48, 622–631 (2006).
Pafundi, D. H. et al. Biopsy validation of 18F-DOPA PET and biodistribution in gliomas for neurosurgical planning and radiotherapy target delineation: results of a prospective pilot study. Neuro Oncol. 15, 1058–1067 (2013).
Ledezma, C. J. et al. 18F-FDOPA PET/MRI fusion in patients with primary/recurrent gliomas: Initial experience. Eur. J. Radiol. 71, 242–248 (2009).
Chen, W. et al. 18F-FDOPA PET imaging of brain tumors: comparison study with 18F-FDG PET and evaluation of diagnostic accuracy. J. Nucl. Med. 47, 904–911 (2006).
Singhal, T., Narayanan, T. K., Jacobs, M. P., Bal, C. & Mantil, J. C. 11C-methionine PET for grading and prognostication in gliomas: a comparison study with 18F-FDG PET and contrast enhancement on MRI. J. Nucl. Med. 53, 1709–1715 (2012).
Jansen, N. L. et al. Dynamic 18F-FET PET in newly diagnosed astrocytic low-grade glioma identifies high-risk patients. J. Nucl. Med. 55, 198–203 (2013).
Galldiks, N. et al. Role of O-(2-18F-fluoroethyl)-l-tyrosine PET as a diagnostic tool for detection of malignant progression in patients with low-grade glioma. J. Nucl. Med. 54, 2046–2054 (2013).
Galldiks, N. et al. Volumetry of [11C]-methionine positron emission tomographic uptake as a prognostic marker before treatment of patients with malignant glioma. Mol. Imaging 11, 516–527 (2012).
Suchorska, B. et al. Biological tumor volume in 18FET-PET before radiochemotherapy correlates with survival in GBM. Neurology 84, 710–719 (2015).
Saraswathy, S. et al. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J. Neurooncol. 91, 69–81 (2008).
Crawford, F. W. et al. Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J. Neurooncol. 91, 337–351 (2008).
Andronesi, O. C. et al. Treatment response assessment in IDH-mutant glioma patients by non-invasive 3D functional spectroscopic mapping of 2-Hydroxyglutarate. Clin. Cancer Res. (2015).
Murphy, P. S. et al. Monitoring temozolomide treatment of low-grade glioma with proton magnetic resonance spectroscopy. Br. J. Cancer 90, 781–786 (2004).
Warren, K. E. et al. Proton magnetic resonance spectroscopic imaging in children with recurrent primary brain tumors. J. Clin. Oncol. 18, 1020–1026 (2000).
Alexander, A. et al. Prognostic significance of serial magnetic resonance spectroscopies over the course of radiation therapy for patients with malignant glioma. Clin. Invest. Med. 29, 301–311 (2006).
Hattingen, E. et al. Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neuro Oncol. 13, 1349–1363 (2011).
Kim, H. et al. Serial magnetic resonance spectroscopy reveals a direct metabolic effect of cediranib in glioblastoma. Cancer Res. 71, 3745–3752 (2011).
Galldiks, N. et al. Use of 11C-methionine PET to monitor the effects of temozolomide chemotherapy in malignant gliomas. Eur. J. Nucl. Med. Mol. Imaging 33, 516–524 (2006).
Masui, K. et al. Glucose-dependent acetylation of Rictor promotes targeted cancer therapy resistance. Proc. Natl Acad. Sci. USA 112, 9406–9411 (2015).
Johnson, B. E. et al. Mutational analysis reveals the origin and therapy-driven evolution of recurrent glioma. Science 343, 189–193 (2014).
Tanaka, K. et al. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J. Clin. Invest. 125, 1591–1602 (2015).
Wen, P. Y. et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J. Clin. Oncol. 28, 1963–1972 (2010).
Okamoto, S. et al. Semiquantitative analysis of C-11 methionine PET may distinguish brain tumor recurrence from radiation necrosis even in small lesions. Ann. Nucl. Med. 25, 213–220 (2010).
Galldiks, N. et al. Diagnosis of pseudoprogression in patients with glioblastoma using O-(2-[18F]fluoroethyl)-l-tyrosine PET. Eur. J. Nucl. Med. Mol. Imaging 42, 685–695 (2015).
Kebir, S. et al. Late pseudoprogression in glioblastoma: diagnostic value of dynamic O-(2-[18F]fluoroethyl)-l-tyrosine PET. Clin. Cancer Res. 22, 2190–2196 (2016).
Herrmann, K. et al. Comparison of visual and semiquantitative analysis of 18F-FDOPA-PET/CT for recurrence detection in glioblastoma patients. Neuro Oncol. 16, 603–609 (2013).
Ullrich, R. T. et al. Methyl-L-11C-methionine PET as a diagnostic marker for malignant progression in patients with glioma. J. Nucl. Med. 50, 1962–1968 (2009).
Galldiks, N. et al. The use of dynamic O-(2-18F-fluoroethyl)-l-tyrosine PET in the diagnosis of patients with progressive and recurrent glioma. Neuro Oncol. 17, 1293–1300 (2015).
Walter, F. et al. Impact of 3,4-dihydroxy-6-18F-fluoro-l-phenylalanine PET/CT on managing patients with brain tumors: the referring physician's perspective. J. Nucl. Med. 53, 393–398 (2012).
Sundgren, P. C. M. R. Spectroscopy in radiation injury. Am. J. Neuroradiol. 30, 1469–1476 (2009).
Rock, J. P. et al. Correlations between magnetic resonance spectroscopy and image-guided histopathology, with special attention to radiation necrosis. Neurosurgery 51, 912–920 (2002).
Horská, A. & Barker, P. B. Imaging of brain tumors: MR spectroscopy and metabolic imaging. Neuroimaging Clin. N. Am. 20, 293–310 (2010).
Hekmatyar, S. K. et al. 1H nuclear magnetic resonance spectroscopy characterisation of metabolic phenotypes in the medulloblastoma of the SMO transgenic mice. Br. J. Cancer 103, 1297–1304 (2010).
Bluml, S. et al. Molecular subgroups of medulloblastoma identification using noninvasive magnetic resonance spectroscopy. Neuro Oncol. 18, 126–131 (2016).
Iwadate, Y., Shinozaki, N., Matsutani, T., Uchino, Y. & Saeki, N. Molecular imaging of 1p/19q deletion in oligodendroglial tumours with 11C-methionine positron emission tomography. J. Neurol. Neurosurg. Psychiatry http://dx.doi.org/10.1136/jnnp-2015-311516 (2016).
Louis, D. N. et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 131, 803–820 (2016).
Ostrom, Q. T. et al. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2008–2012. Neuro Oncol. 17 (Suppl. 4), iv1–iv62 (2015).
Acknowledgements
We thanks Prof. Yue Cao of the Functional Imaging Group in the Department of Radiation Oncology at the University of Michigan Health System, Ann Arbor, Michigan, USA, for providing the imaging scans included in Fig. 6. The work of S.V. is supported by the US NIH National Cancer Institute (grant K08CA181475A), the Mathew Larson Foundation, the Sidney Kimmel Foundation and the Doris Duke Foundation.
Author information
Authors and Affiliations
Contributions
M.M.K. and S.V. researched the data for the article and wrote the manuscript M.M.K., A.P., and S.V. contributed substantially to discussions of the content. M.P.D. contributed figures. All authors reviewed and/or edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Kim, M., Parolia, A., Dunphy, M. et al. Non-invasive metabolic imaging of brain tumours in the era of precision medicine. Nat Rev Clin Oncol 13, 725–739 (2016). https://doi.org/10.1038/nrclinonc.2016.108
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2016.108
This article is cited by
-
Single-cell mapping of lipid metabolites using an infrared probe in human-derived model systems
Nature Communications (2024)
-
A bis-boron boramino acid PET tracer for brain tumor diagnosis
European Journal of Nuclear Medicine and Molecular Imaging (2024)
-
Clinical application of magnetic resonance elastography in pediatric neurological disorders
Pediatric Radiology (2023)
-
Detection of recurrent high-grade glioma using microstructure characteristics of distinct metabolic compartments in a multimodal and integrative 18F-FET PET/fast-DKI approach
European Radiology (2023)
-
Feasibility of [18F]fluoropivalate hybrid PET/MRI for imaging lower and higher grade glioma: a prospective first-in-patient pilot study
European Journal of Nuclear Medicine and Molecular Imaging (2023)