Key Points
-
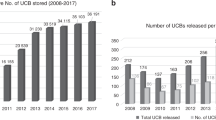
Umbilical cord blood (UCB) transplantation is limited by cell doses, especially for adult recipients
-
UCB can be expanded in vitro greater than 500-fold
-
UCB can be 'primed' just before infusion to influence cellular homing
-
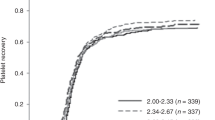
Cell expansion or modulation of cell homing can alter engraftment kinetics in recipients
-
These strategies can increase the rapidity of engraftment and the next generation of clinical trials will determine the clinical efficacy of such approaches
Abstract
The use of umbilical cord blood (UCB) as an alternative haematopoietic cell source in lieu of bone marrow for haematopoietic reconstitution is increasingly becoming a mainstay treatment for both malignant and nonmalignant diseases, as most individuals will have at least one available, suitably HLA-matched unit of blood. The principal limitation of UCB is the low and finite number of haematopoietic stem and progenitor cells (HSPC) relative to the number found in a typical bone marrow or mobilized peripheral blood allograft, which leads to prolonged engraftment times. In an attempt to overcome this obstacle, strategies that are often based on native processes occurring in the bone marrow microenvironment or 'niche' have been developed with the goal of accelerating UCB engraftment. In broad terms, the two main approaches have been either to expand UCB HSPC ex vivo before transplantation, or to modulate HSPC functionality to increase the efficiency of HSPC homing to the bone marrow niche after transplant both of which enhance the biological activities of the engrafted HSPC. Several early phase clinical trials of these approaches have reported promising results.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kita, K., Lee, J. O., Finnerty, C. C. & Herndon, D. N. Cord blood-derived hematopoietic stem/progenitor cells: current challenges in engraftment, infection, and ex vivo expansion. Stem Cells Int. 2011, 276193 (2011).
Laughlin, M. J. et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N. Engl. J. Med. 351, 2265–2275 (2004).
Ballen, K. K., Gluckman, E. & Broxmeyer, H. E. Umbilical cord blood transplantation: the first 25 years and beyond. Blood 122, 491–498 (2013).
Broxmeyer, H. E. et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc. Natl Acad. Sci. USA 86, 3828–3832 (1989).
Calvet, L. et al. Hematologic, immunologic reconstitution, and outcome of 342 autologous peripheral blood stem cell transplantations after cryopreservation in a −80 degrees C mechanical freezer and preserved less than 6 months. Transfusion 53, 570–578 (2013).
Davies, S. M. et al. Engraftment and survival after unrelated-donor bone marrow transplantation: a report from the national marrow donor program. Blood 96, 4096–4102 (2000).
Eapen, M. et al. Higher mortality after allogeneic peripheral-blood transplantation compared with bone marrow in children and adolescents: the Histocompatibility and Alternate Stem Cell Source Working Committee of the International Bone Marrow Transplant Registry. J. Clin. Oncol. 22, 4872–4880 (2004).
Rubinstein, P. et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N. Engl. J. Med. 339, 1565–1577 (1998).
Anasetti, C. et al. Peripheral-blood stem cells versus bone marrow from unrelated donors. N. Engl. J. Med. 367, 1487–1496 (2012).
Avery, S. et al. Influence of infused cell dose and HLA match on engraftment after double-unit cord blood allografts. Blood 117, 3277–3285 (2011).
Laughlin, M. J. et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N. Engl. J. Med. 344, 1815–1822 (2001).
Dexter, T. M., Allen, T. D. & Lajtha, L. G. Conditions controlling the proliferation of haemopoietic stem cells in vitro. J. Cell. Physiol. 91, 335–344 (1977).
Broxmeyer, H. E. et al. Growth characteristics and expansion of human umbilical cord blood and estimation of its potential for transplantation in adults. Proc. Natl Acad. Sci. USA 89, 4109–4113 (1992).
Gabutti, V. et al. Expansion of cord blood progenitors and use for hemopoietic reconstitution. Stem Cells 11 (Suppl. 2), 105–112 (1993).
Mayani, H., Dragowska, W. & Lansdorp, P. M. Cytokine-induced selective expansion and maturation of erythroid versus myeloid progenitors from purified cord blood precursor cells. Blood 81, 3252–3258 (1993).
Migliaccio, A. R., Migliaccio, G., Durand, B., Mancini, G. C. & Adamson, J. W. The generation of colony-forming cells (CFC) and the expansion of hematopoiesis in cultures of human cord blood cells is dependent on the presence of stem cell factor (SCF). Cytotechnology 11, 107–113 (1993).
Brugger, W., Scheding, S., Ziegler, B., Buhring, H. J. & Kanz, L. Ex vivo manipulation of hematopoietic stem and progenitor cells. Semin. Hematol. 37, 42–49 (2000).
Shpall, E. J. et al. Transplantation of ex vivo expanded cord blood. Biol. Blood Marrow Transplant. 8, 368–376 (2002).
Lee, S. J. et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood 110, 4576–4583 (2007).
Zidar, B. L., Shadduck, R. K., Zeigler, Z. & Winkelstein, A. Observations on the anemia and neutropenia of human copper deficiency. Am. J. Hematol. 3, 177–185 (1977).
Percival, S. S. Neutropenia caused by copper deficiency: possible mechanisms of action. Nutr. Rev. 53, 59–66 (1995).
Peled, T. et al. Cellular copper content modulates differentiation and self-renewal in cultures of cord blood-derived CD34+ cells. Br. J. Haematol. 116, 655–661 (2002).
Peled, T. et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp. Hematol. 32, 547–555 (2004).
de Lima, M. et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant. 41, 771–778 (2008).
Barker, J. N., Weisdorf, D. J. & Wagner, J. E. Creation of a double chimera after the transplantation of umbilical-cord blood from two partially matched unrelated donors. N. Engl. J. Med. 344, 1870–1871 (2001).
Brunstein, C. G. et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 116, 4693–4699 (2010).
Scaradavou, A. et al. Double unit grafts successfully extend the application of umbilical cord blood transplantation in adults with acute leukemia. Blood 121, 752–758 (2013).
Wagner, J. E. Should double cord blood transplants be the preferred choice when a sibling donor is unavailable? Best Pract. Res. Clin. Haematol. 22, 551–555 (2009).
Verneris, M. R. et al. Relapse risk after umbilical cord blood transplantation: enhanced graft-versus-leukemia effect in recipients of 2 units. Blood 114, 4293–4299 (2009).
Ramirez, P. et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 47, 799–803 (2012).
Cutler, C. et al. Donor-specific anti-HLA antibodies predict outcome in double umbilical cord blood transplantation. Blood 118, 6691–6697 (2011).
Barker, J. N. et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 105, 1343–1347 (2005).
Haspel, R. L. et al. Preinfusion variables predict the predominant unit in the setting of reduced-intensity double cord blood transplantation. Bone Marrow Transplant. 41, 523–529 (2008).
Somers, J. A. et al. Double umbilical cord blood transplantation: a study of early engraftment kinetics in leukocyte subsets using HLA-specific monoclonal antibodies. Biol. Blood Marrow Transplant. 19, 266–273 (2013).
Andersson, E. R., Sandberg, R. & Lendahl, U. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612 (2011).
Sethi, N. & Kang, Y. Notch signalling in cancer progression and bone metastasis. Br. J. Cancer 105, 1805–1810 (2011).
Milner, L. A., Kopan, R., Martin, D. I. & Bernstein, I. D. A human homologue of the Drosophila developmental gene, Notch, is expressed in CD34+ hematopoietic precursors. Blood 83, 2057–2062 (1994).
Varnum-Finney, B. et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat. Med. 6, 1278–1281 (2000).
Varnum-Finney, B. et al. Immobilization of Notch ligand, Delta-1, is required for induction of notch signaling. J. Cell Sci. 113, 4313–4318 (2000).
Dallas, M. H., Varnum-Finney, B., Martin, P. J. & Bernstein, I. D. Enhanced T-cell reconstitution by hematopoietic progenitors expanded ex vivo using the Notch ligand Delta1. Blood 109, 3579–3587 (2007).
Varnum-Finney, B., Brashem-Stein, C. & Bernstein, I. D. Combined effects of Notch signaling and cytokines induce a multiple log increase in precursors with lymphoid and myeloid reconstituting ability. Blood 101, 1784–1789 (2003).
Delaney, C., Varnum-Finney, B., Aoyama, K., Brashem-Stein, C. & Bernstein, I. D. Dose-dependent effects of the Notch ligand Delta1 on ex vivo differentiation and in vivo marrow repopulating ability of cord blood cells. Blood 106, 2693–2699 (2005).
Delaney, C. et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat. Med. 16, 232–236 (2010).
Prockop, D. J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 276, 71–74 (1997).
Musina, R. A., Bekchanova, E. S. & Sukhikh, G. T. Comparison of mesenchymal stem cells obtained from different human tissues. Bull. Exp. Biol. Med. 139, 504–509 (2005).
Crisan, M. et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 3, 301–313 (2008).
Corselli, M., Chen, C. W., Crisan, M., Lazzari, L. & Peault, B. Perivascular ancestors of adult multipotent stem cells. Arterioscler. Thromb. Vasc. Biol. 30, 1104–1109 (2010).
Weber, J. M. & Calvi, L. M. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone 46, 281–285 (2010).
Caplan, A. I. & Dennis, J. E. Mesenchymal stem cells as trophic mediators. J. Cell. Biochem. 98, 1076–1084 (2006).
Robinson, S. N. et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 37, 359–366 (2006).
McNiece, I., Harrington, J., Turney, J., Kellner, J. & Shpall, E. J. Ex vivo expansion of cord blood mononuclear cells on mesenchymal stem cells. Cytotherapy 6, 311–317 (2004).
de Lima, M. et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N. Engl. J. Med. 367, 2305–2315 (2012).
Tang, B. L. Sirt1's systemic protective roles and its promise as a target in antiaging medicine. Transl. Res. 157, 276–284 (2011).
Borradaile, N. M. & Pickering, J. G. NAD+, sirtuins, and cardiovascular disease. Curr. Pharm. Des. 15, 110–117 (2009).
Narala, S. R. et al. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol. Biol. Cell 19, 1210–1219 (2008).
Ou, X. et al. SIRT1 deficiency compromises mouse embryonic stem cell hematopoietic differentiation, and embryonic and adult hematopoiesis in the mouse. Blood 117, 440–450 (2011).
Peled, T. et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp. Hematol. 40, 342–355.e1 (2012).
Fruscione, F. et al. Regulation of human mesenchymal stem cell functions by an autocrine loop involving NAD+ release and P2Y11-mediated signaling. Stem Cells Dev. 20, 1183–1198 (2011).
Li, Y., He, J., He, X., Li, Y. & Lindgren, U. Nampt expression increases during osteogenic differentiation of multi- and omnipotent progenitors. Biochem. Biophys. Res. Commun. 434, 117–123 (2013).
Horwitz, M. E. et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J. Clin. Invest. 124, 3121–3128 (2014).
Boitano, A. E. et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 329, 1345–1348 (2010).
Dvorak, Z., Vrzal, R., Starha, P., Klanicova, A. & Travnicek, Z. Effects of dinuclear copper(II) complexes with 6-(benzylamino)purine derivatives on AhR and PXR dependent expression of cytochromes P450 CYP1A2 and CYP3A4 genes in primary cultures of human hepatocytes. Toxicol. In Vitro 24, 425–429 (2010).
van Os, R. et al. Engraftment of syngeneic bone marrow is not more efficient after intrafemoral transplantation than after traditional intravenous administration. Exp. Hematol. 38, 1115–1123 (2010).
Castello, S. et al. Intra-bone marrow injection of bone marrow and cord blood cells: an alternative way of transplantation associated with a higher seeding efficiency. Exp. Hematol. 32, 782–787 (2004).
Brunstein, C. G. et al. Intra-BM injection to enhance engraftment after myeloablative umbilical cord blood transplantation with two partially HLA-matched units. Bone Marrow Transplant. 43, 935–940 (2009).
Frassoni, F. et al. The intra-bone marrow injection of cord blood cells extends the possibility of transplantation to the majority of patients with malignant hematopoietic diseases. Best Pract. Res. Clin. Haematol. 23, 237–244 (2010).
Rocha, V. et al. Unrelated cord blood transplantation: outcomes after single-unit intrabone injection compared with double-unit intravenous injection in patients with hematological malignancies. Transplantation 95, 1284–1291 (2013).
Tashiro, K. et al. Signal sequence trap: a cloning strategy for secreted proteins and type I membrane proteins. Science 261, 600–603 (1993).
Kim, C. H. & Broxmeyer, H. E. In vitro behavior of hematopoietic progenitor cells under the influence of chemoattractants: stromal cell-derived factor-1, steel factor, and the bone marrow environment. Blood 91, 100–110 (1998).
Aiuti, A., Webb, I. J., Bleul, C., Springer, T. & Gutierrez-Ramos, J. C. The chemokine SDF-1 is a chemoattractant for human CD34+ hematopoietic progenitor cells and provides a new mechanism to explain the mobilization of CD34+ progenitors to peripheral blood. J. Exp. Med. 185, 111–120 (1997).
Bleul, C. C., Fuhlbrigge, R. C., Casasnovas, J. M., Aiuti, A. & Springer, T. A. A highly efficacious lymphocyte chemoattractant, stromal cell-derived factor 1 (SDF-1). J. Exp. Med. 184, 1101–1109 (1996).
Peled, A. et al. Dependence of human stem cell engraftment and repopulation of NOD/SCID mice on CXCR4. Science 283, 845–848 (1999).
Christopherson, K. W. 2nd, Paganessi, L. A., Napier, S. & Porecha, N. K. CD26 inhibition on CD34+ or lineage− human umbilical cord blood donor hematopoietic stem cells/hematopoietic progenitor cells improves long-term engraftment into NOD/SCID/Beta2null immunodeficient mice. Stem Cells Dev. 16, 355–360 (2007).
Broxmeyer, H. E. et al. Dipeptidylpeptidase 4 negatively regulates colony-stimulating factor activity and stress hematopoiesis. Nat. Med. 18, 1786–1796 (2012).
Christopherson, K. W. 2nd, Hangoc, G. & Broxmeyer, H. E. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J. Immunol. 169, 7000–7008 (2002).
Christopherson, K. W. 2nd, Hangoc, G., Mantel, C. R. & Broxmeyer, H. E. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 305, 1000–1003 (2004).
Farag, S. S. et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev. 22, 1007–1015 (2013).
Ratajczak, M. Z., Kim, C., Ratajczak, J. & Janowska-Wieczorek, A. Innate immunity as orchestrator of bone marrow homing for hematopoietic stem/progenitor cells. Adv. Exp. Med. Biol. 735, 219–232 (2013).
Reca, R. et al. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood 101, 3784–3793 (2003).
Wysoczynski, M. et al. Defective engraftment of C3aR−/− hematopoietic stem progenitor cells shows a novel role of the C3a-C3aR axis in bone marrow homing. Leukemia 23, 1455–1461 (2009).
Ratajczak, J. et al. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood 103, 2071–2078 (2004).
Brunstein, C. G. et al. Complement fragment 3a priming of umbilical cord blood progenitors: safety profile. Biol. Blood Marrow Transplant. 19, 1474–1479 (2013).
North, T. E. et al. Prostaglandin E2 regulates vertebrate haematopoietic stem cell homeostasis. Nature 447, 1007–1011 (2007).
Yagi, H. et al. Mesenchymal stem cells: mechanisms of immunomodulation and homing. Cell Transplant. 19, 667–679 (2010).
Hoggatt, J., Singh, P., Sampath, J. & Pelus, L. M. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 113, 5444–5455 (2009).
Aggarwal, S. & Pittenger, M. F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105, 1815–1822 (2005).
English, K. et al. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25High forkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 156, 149–160 (2009).
DeGowin, R. L. & Gibson, D. P. Prostaglandin-mediated enhancement of erythroid colonies by marrow stromal cells (MSC). Exp. Hematol. 9, 274–280 (1981).
Cutler, C. et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 122, 3074–3081 (2013).
Jetmore, A. et al. Homing efficiency, cell cycle kinetics, and survival of quiescent and cycling human CD34+ cells transplanted into conditioned NOD/SCID recipients. Blood 99, 1585–1593 (2002).
Orschell-Traycoff, C. M. et al. Homing and engraftment potential of Sca-1+lin− cells fractionated on the basis of adhesion molecule expression and position in cell cycle. Blood 96, 1380–1387 (2000).
Geyer, M. B. et al. T cell depletion utilizing CD34+ stem cell selection and CD3+ addback from unrelated adult donors in paediatric allogeneic stem cell transplantation recipients. Br. J. Haematol. 157, 205–219 (2012).
Lang, P. et al. Transplantation of highly purified peripheral-blood CD34+ progenitor cells from related and unrelated donors in children with nonmalignant diseases. Bone Marrow Transplant. 33, 25–32 (2004).
Peters, C. et al. Transplantation of highly purified peripheral blood CD34+ cells from HLA-mismatched parental donors in 14 children: evaluation of early monitoring of engraftment. Leukemia 13, 2070–2078 (1999).
Arber, C. et al. Protection against lethal Aspergillus fumigatus infection in mice by allogeneic myeloid progenitors is not major histocompatibility complex restricted. J. Infect. Dis. 192, 1666–1671 (2005).
Arber, C. et al. Common lymphoid progenitors rapidly engraft and protect against lethal murine cytomegalovirus infection after hematopoietic stem cell transplantation. Blood 102, 421–428 (2003).
BitMansour, A. et al. Myeloid progenitors protect against invasive aspergillosis and Pseudomonas aeruginosa infection following hematopoietic stem cell transplantation. Blood 100, 4660–4667 (2002).
Broxmeyer, H. E. & Pelus, L. M. Inhibition of DPP4/CD26 and dmPGE2 treatment enhances engraftment of mouse bone marrow hematopoietic stem cells. Blood Cells Mol. Dis. 53, 34–38 (2014).
Barker, J. N., Byam, C. & Scaradavou, A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood 117, 2332–2339 (2011).
Wagner, J. E. et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N. Engl. J. Med. 371, 1685–1694 (2014).
Wahid, S. F. Indications and outcomes of reduced-toxicity hematopoietic stem cell transplantation in adult patients with hematological malignancies. Int. J. Hematol. 97, 581–598 (2013).
Bart, T. Cost effectiveness of cord blood versus bone marrow and peripheral blood stem cells. Clinicoecon. Outcomes Res. 2, 141–147 (2010).
Majhail, N. S. et al. Costs of pediatric allogeneic hematopoietic-cell transplantation. Pediatr. Blood Cancer 54, 138–143 (2010).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2014).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
US National Library of Medicine. ClinicalTrials.gov[online], (2013).
Acknowledgements
The authors thank T. Glass for her conception and rendition of the bone-marrow microenvironment in Figure 2.
Author information
Authors and Affiliations
Contributions
T.C.L. researched data for article. A.E.B., C.S.D., E.J.S. and J.E.W. reviewed and edited the manuscript before submission. T.C.L. and J.E.W. substantially contributed to discussion of content. T.C.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Lund, T., Boitano, A., Delaney, C. et al. Advances in umbilical cord blood manipulation—from niche to bedside. Nat Rev Clin Oncol 12, 163–174 (2015). https://doi.org/10.1038/nrclinonc.2014.215
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2014.215
This article is cited by
-
Evolving therapies in neuronopathic LSDs: opportunities and challenges
Metabolic Brain Disease (2022)
-
Cord blood research, banking, and transplantation: achievements, challenges, and perspectives
Bone Marrow Transplantation (2020)
-
GvHD after umbilical cord blood transplantation for acute leukemia: an analysis of risk factors and effect on outcomes
Bone Marrow Transplantation (2017)
-
Quality cell therapy manufacturing by design
Nature Biotechnology (2016)
-
Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells
Stem Cell Reviews and Reports (2016)