Key Points
-
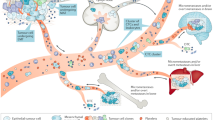
Circulating tumour cells (CTCs) can be captured from a simple blood test and their molecular characterization can provide vital insights into tumour heterogeneity
-
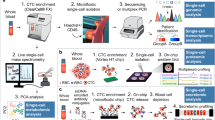
Advances in single-cell genomic profiling have enhanced the ability to interrogate CTCs for 'actionable' aberrations and emerging resistant subclones, which will assist in patient treatment stratification
-
Biological processes during CTC invasion and metastasis such as epithelial–mesenchymal transition and circulating tumour microemboli, typically explored in preclinical models are now being examined in the clinical setting
-
CTC and cell-free DNA mutational profiling represent complementary biomarkers for identification of predictive targets and real-time monitoring of disease in clinical practice
-
CTC assay validation and clinical qualification is required if CTCs are to become routine tests in the clinic; collaborative efforts between academia and industry are needed to make this a reality
Abstract
Growing evidence for intratumour heterogeneity informs us that single-site biopsies fall short of revealing the complete genomic landscape of a tumour. With an expanding repertoire of targeted agents entering the clinic, screening tumours for genomic aberrations is increasingly important, as is interrogating the tumours for resistance mechanisms upon disease progression. Multiple biopsies separated spatially and temporally are impractical, uncomfortable for the patient and not without risk. Here, we describe how circulating tumour cells (CTCs), captured from a minimally invasive blood test—and readily amenable to serial sampling—have the potential to inform intratumour heterogeneity and tumour evolution, although it remains to be determined how useful this will be in the clinic. Technologies for detecting and isolating CTCs include the validated CellSearch® system, but other technologies are gaining prominence. We also discuss how recent CTC discoveries map to mechanisms of haematological spread, previously described in preclinical models, including evidence for epithelial–mesenchymal transition, collective cell migration and cells with tumour-initiating capacity within the circulation. Advances in single-cell molecular analysis are enhancing our ability to explore mechanisms of metastasis, and the combination of CTC and cell-free DNA assays are anticipated to provide invaluable blood-borne biomarkers for real-time patient monitoring and treatment stratification.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Schilsky, R. L. Personalized medicine in oncology: the future is now. Nat. Rev. Drug Discov. 9, 363–366 (2010).
McDermott, U. & Settleman, J. Personalized cancer therapy with selective kinase inhibitors: an emerging paradigm in medical oncology. J. Clin. Oncol. 27, 5650–5659 (2009).
Dancey, J. E., Bedard, P. L., Onetto, N. & Hudson, T. J. The genetic basis for cancer treatment decisions. Cell 148, 409–420 (2012).
Dienstmann, R., Rodon, J. & Tabernero, J. Biomarker-driven patient selection for early clinical trials. Curr. Opin. Oncol. 25, 305–312 (2013).
Shaw, A. T. et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N. Engl. J. Med. 368, 2385–2394 (2013).
Zhou, C. et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 12, 735–742 (2011).
Mok, T. S. et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N. Engl. J. Med. 361, 947–957 (2009).
Pao, W. et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2, e73 (2005).
Pao, W. et al. KRAS mutations and primary resistance of lung adenocarcinomas to gefitinib or erlotinib. PLoS Med. 2, e17 (2005).
Garraway, L. A. & Baselga, J. Whole-genome sequencing and cancer therapy: is too much ever enough? Cancer Discov. 2, 766–768 (2012).
Gonzalez de Castro, D., Clarke, P. A., Al-Lazikani, B. & Workman, P. Personalized cancer medicine: molecular diagnostics, predictive biomarkers, and drug resistance. Clin. Pharmacol. Ther. 93, 252–259 (2013).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012).
Wu, C. C., Maher, M. M. & Shepard, J. A. Complications of CT-guided percutaneous needle biopsy of the chest: prevention and management. AJR Am. J. Roentgenol. 196, W678–W682 (2011).
Yachida, S. et al. Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467, 1114–1117 (2010).
Gerlinger, M. et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N. Engl. J. Med. 366, 883–892 (2012).
Ding, L. et al. Genome remodeling in a basal-like breast cancer metastasis and xenograft. Nature 464, 999–1005 (2010).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Greaves, M. F., Maia, A. T., Wiemels, J. L. & Ford, A. M. Leukemia in twins: lessons in natural history. Blood 102, 2321–2333 (2003).
Bateman, C. M. et al. Acquisition of genome-wide copy number alterations in monozygotic twins with acute lymphoblastic leukemia. Blood 115, 3553–3558 (2010).
Anderson, K. et al. Genetic variegation of clonal architecture and propagating cells in leukaemia. Nature 469, 356–361 (2011).
Landau, D. A. et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell 152, 714–726 (2013).
Swanton, C. Intratumor heterogeneity: evolution through space and time. Cancer Res. 72, 4875–4882 (2012).
Fidler, I. J. Tumor heterogeneity and the biology of cancer invasion and metastasis. Cancer Res. 38, 2651–2660 (1978).
Greaves, M. & Maley, C. C. Clonal evolution in cancer. Nature 481, 306–313 (2012).
Kim, M. Y. et al. Tumor self-seeding by circulating cancer cells. Cell 139, 1315–1326 (2009).
Pantel, K. & Brakenhoff, R. H. Dissecting the metastatic cascade. Nat. Rev. Cancer 4, 448–456 (2004).
Baccelli, I. et al. Identification of a population of blood circulating tumor cells from breast cancer patients that initiates metastasis in a xenograft assay. Nat. Biotechnol. 31, 539–544 (2013).
Yu, M., Stott, S., Toner, M., Maheswaran, S. & Haber, D. A. Circulating tumor cells: approaches to isolation and characterization. J. Cell Biol. 192, 373–382 (2011).
Alix-Panabières, C. & Pantel, K. Circulating tumor cells: liquid biopsy of cancer. Clin. Chem. 59, 110–118 (2013).
Hou, J. M. et al. Clinical significance and molecular characteristics of circulating tumor cells and circulating tumor microemboli in patients with small-cell lung cancer. J. Clin. Oncol. 30, 525–532 (2012).
Krebs, M. G. et al. Evaluation and prognostic significance of circulating tumor cells in patients with non-small-cell lung cancer. J. Clin. Oncol. 29, 1556–1563 (2011).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Cohen, S. J. et al. Relationship of circulating tumor cells to tumor response, progression-free survival, and overall survival in patients with metastatic colorectal cancer. J. Clin. Oncol. 26, 3213–3221 (2008).
Cristofanilli, M. et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N. Engl. J. Med. 351, 781–791 (2004).
Khan, M. S. et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J. Clin. Oncol. 31, 365–372 (2013).
Khoja, L. et al. Biomarker utility of circulating tumor cells in metastatic cutaneous melanoma. J. Invest. Dermatol. 133, 1582–1590 (2013).
Hiraiwa, K. et al. Clinical significance of circulating tumor cells in blood from patients with gastrointestinal cancers. Ann. Surg. Oncol. 15, 3092–3100 (2008).
Devriese, L. A., Voest, E. E., Beijnen, J. H. & Schellens, J. H. Circulating tumor cells as pharmacodynamic biomarker in early clinical oncological trials. Cancer Treat. Rev. 37, 579–589 (2011).
Pailler, E. et al. Detection of circulating tumor cells harboring a unique ALK rearrangement in ALK-positive non-small-cell lung cancer. J. Clin. Oncol. 31, 2273–2281 (2013).
Maheswaran, S. et al. Detection of mutations in EGFR in circulating lung-cancer cells. N. Engl. J. Med. 359, 366–377 (2008).
Pestrin, M. et al. Correlation of HER2 status between primary tumors and corresponding circulating tumor cells in advanced breast cancer patients. Breast Cancer Res. Treat. 118, 523–530 (2009).
Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918 (2009).
Schneck, H. et al. Analysing the mutational status of PIK3CA in circulating tumor cells from metastatic breast cancer patients. Mol. Oncol. 7, 976–986 (2013).
Zhang, L. et al. The identification and characterization of breast cancer CTCs competent for brain metastasis. Sci. Transl. Med. 5, 180ra148 (2013).
Tang, F. et al. mRNA-Seq whole-transcriptome analysis of a single cell. Nat. Methods 6, 377–382 (2009).
Ramsköld, D. et al. Full-length mRNA-Seq from single-cell levels of RNA and individual circulating tumor cells. Nat. Biotechnol. 30, 777–782 (2012).
Thiery, J. P. Epithelial–mesenchymal transitions in tumour progression. Nat. Rev. Cancer 2, 442–454 (2002).
Thiery, J. P. & Lim, C. T. Tumor dissemination: an EMT affair. Cancer Cell 23, 272–273 (2013).
Tsuji, T., Ibaragi, S. & Hu, G. F. Epithelial–mesenchymal transition and cell cooperativity in metastasis. Cancer Res. 69, 7135–7139 (2009).
Tsuji, T. et al. Epithelial–mesenchymal transition induced by growth suppressor p12CDK2–AP1 promotes tumor cell local invasion but suppresses distant colony growth. Cancer Res. 68, 10377–10386 (2008).
Calbo, J. et al. A functional role for tumor cell heterogeneity in a mouse model of small cell lung cancer. Cancer Cell 19, 244–256 (2011).
Duda, D. G. et al. Malignant cells facilitate lung metastasis by bringing their own soil. Proc. Natl Acad. Sci. USA 107, 21677–21682 (2010).
Friedl, P. et al. Migration of coordinated cell clusters in mesenchymal and epithelial cancer explants in vitro. Cancer Res. 55, 4557–4560 (1995).
Bednarz-Knoll, N., Alix-Panabières, C. & Pantel, K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res. 13, 228 (2011).
Vona, G. et al. Isolation by size of epithelial tumor cells: a new method for the immunomorphological and molecular characterization of circulating tumor cells. Am. J. Pathol. 156, 57–63 (2000).
Müller, V. et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin. Cancer Res. 11, 3678–3685 (2005).
Gascoyne, P. R., Noshari, J., Anderson, T. J. & Becker, F. F. Isolation of rare cells from cell mixtures by dielectrophoresis. Electrophoresis 30, 1388–1398 (2009).
Moon, H. S. et al. Continuous separation of breast cancer cells from blood samples using multi-orifice flow fractionation (MOFF) and dielectrophoresis (DEP). Lab Chip 11, 1118–1125 (2011).
Allard, W. J. et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin. Cancer Res. 10, 6897–6904 (2004).
Riethdorf, S. et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin. Cancer Res. 13, 920–928 (2007).
Talasaz, A. H. et al. Isolating highly enriched populations of circulating epithelial cells and other rare cells from blood using a magnetic sweeper device. Proc. Natl Acad. Sci. USA 106, 3970–3975 (2009).
Saucedo-Zeni, N. et al. A novel method for the in vivo isolation of circulating tumor cells from peripheral blood of cancer patients using a functionalized and structured medical wire. Int. J. Oncol. 41, 1241–1250 (2012).
Nagrath, S. et al. Isolation of rare circulating tumour cells in cancer patients by microchip technology. Nature 450, 1235–1239 (2007).
Stott, S. L. et al. Isolation of circulating tumor cells using a microvortex-generating herringbone-chip. Proc. Natl Acad. Sci. USA 107, 18392–18397 (2010).
Ozkumur, E. et al. Inertial focusing for tumor antigen-dependent and -independent sorting of rare circulating tumor cells. Sci. Transl. Med. 5, 179ra147 (2013).
Saliba, A. E. et al. Microfluidic sorting and multimodal typing of cancer cells in self-assembled magnetic arrays. Proc. Natl Acad. Sci. USA 107, 14524–14529 (2010).
Zieglschmid, V. et al. Combination of immunomagnetic enrichment with multiplex RT-PCR analysis for the detection of disseminated tumor cells. Anticancer Res. 25, 1803–1810 (2005).
Harb, W. et al. Mutational analysis of circulating tumor cells using a novel microfluidic collection device and qPCR assay. Transl. Oncol. 6, 528–538 (2013).
Vona, G. et al. Impact of cytomorphological detection of circulating tumor cells in patients with liver cancer. Hepatology 39, 792–797 (2004).
Hou, H. W. et al. Isolation and retrieval of circulating tumor cells using centrifugal forces. Sci. Rep. 3, 1259 (2013).
Alix-Panabieres, C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 195, 69–76 (2012).
Lu, J. et al. Isolation of circulating epithelial and tumor progenitor cells with an invasive phenotype from breast cancer patients. Int. J. Cancer 126, 669–683 (2010).
López-Riquelme, N. et al. Imaging cytometry for counting circulating tumor cells: comparative analysis of the CellSearch vs Image Stream systems. APMIS http://dx.doi.org/10.1111/apm.12061.
Marrinucci, D. et al. Fluid biopsy in patients with metastatic prostate, pancreatic and breast cancers. Phys. Biol. 9, 016003 (2012).
Kraan, J. et al. External quality assurance of circulating tumor cell enumeration using the CellSearch® system: a feasibility study. Cytometry B Clin. Cytom. 80, 112–118 (2011).
Parkinson, D. R. et al. Considerations in the development of circulating tumor cell technology for clinical use. J. Transl. Med. 10, 138 (2012).
Stathopoulou, A. et al. Real-time quantification of CK-19 mRNA-positive cells in peripheral blood of breast cancer patients using the lightcycler system. Clin. Cancer Res. 9, 5145–5151 (2003).
Xenidis, N. et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J. Clin. Oncol. 27, 2177–2184 (2009).
Iinuma, H. et al. Clinical significance of circulating tumor cells, including cancer stem-like cells, in peripheral blood for recurrence and prognosis in patients with Dukes' stage B and C colorectal cancer. J. Clin. Oncol. 29, 1547–1555 (2011).
Chimonidou, M., Kallergi, G., Georgoulias, V., Welch, D. R. & Lianidou, E. S. Breast cancer metastasis suppressor-1 promoter methylation provides prognostic information in primary breast tumors. Mol. Cancer Res. 11, 1248–1257 (2013).
Chen, C. L. et al. Single-cell analysis of circulating tumor cells identifies cumulative expression patterns of EMT-related genes in metastatic prostate cancer. Prostate 73, 813–826 (2013).
Fabbri, F. et al. Detection and recovery of circulating colon cancer cells using a dielectrophoresis-based device: KRAS mutation status in pure CTCs. Cancer Lett. 335, 225–231 (2013).
Peeters, D. J. et al. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. Br. J. Cancer 108, 1358–1367 (2013).
Punnoose, E. A. et al. Evaluation of circulating tumor cells and circulating tumor DNA in non-small cell lung cancer: association with clinical endpoints in a phase II clinical trial of pertuzumab and erlotinib. Clin. Cancer Res. 18, 2391–2401 (2012).
Gasch, C. et al. Heterogeneity of epidermal growth factor receptor status and mutations of KRAS/PIK3CA in circulating tumor cells of patients with colorectal cancer. Clin. Chem. 59, 252–260 (2013).
Voet, T. et al. Single-cell paired-end genome sequencing reveals structural variation per cell cycle. Nucleic Acids Res. 41, 6119–6138 (2013).
Dean, F. B. et al. Comprehensive human genome amplification using multiple displacement amplification. Proc. Natl Acad. Sci. USA 99, 5261–5266 (2002).
De Roock, W. et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. 11, 753–762 (2010).
Klein, C. A. et al. Combined transcriptome and genome analysis of single micrometastatic cells. Nat. Biotechnol. 20, 387–392 (2002).
Ulmer, A. et al. Immunomagnetic enrichment, genomic characterization, and prognostic impact of circulating melanoma cells. Clin. Cancer Res. 10, 531–537 (2004).
Heitzer, E. et al. Complex tumor genomes inferred from single circulating tumor cells by array-CGH and next-generation sequencing. Cancer Res. 73, 2965–2975 (2013).
Powell, A. A. et al. Single cell profiling of circulating tumor cells: transcriptional heterogeneity and diversity from breast cancer cell lines. PLoS ONE 7, e33788 (2012).
Cann, G. M. et al. mRNA-Seq of single prostate cancer circulating tumor cells reveals recapitulation of gene expression and pathways found in prostate cancer. PLoS ONE 7, e49144 (2012).
Asare, A. L. et al. Differential gene expression profiles are dependent upon method of peripheral blood collection and RNA isolation. BMC Genomics 9, 474 (2008).
Fidler, I. J. The relationship of embolic homogeneity, number, size and viability to the incidence of experimental metastasis. Eur. J. Cancer 9, 223–227 (1973).
Liotta, L. A., Kleinerman, J. & Saidel, G. M. Quantitative relationships of intravascular tumor cells, tumor vessels, and pulmonary metastases following tumor implantation. Cancer Res. 34, 997–1004 (1974).
Glaves, D. Correlation between circulating cancer cells and incidence of metastases. Br. J. Cancer 48, 665–673 (1983).
Liotta, L. A., Saidel, M. G. & Kleinerman, J. The significance of hematogenous tumor cell clumps in the metastatic process. Cancer Res. 36, 889–894 (1976).
Watanabe, S. The metastasizability of tumor cells. Cancer 7, 215–223 (1954).
Garvie, W. H. & Matheson, A. B. The effect of intravenous fluids on the development on experimental tumour metastases: their effect on tumour cell aggregation. Br. J. Cancer 20, 838–846 (1966).
Lione, A. & Bosmann, H. B. Quantitative relationship between volume of tumour cell units and their intravascular survival. Br. J. Cancer 37, 248–253 (1978).
Topal, B., Roskams, T., Fevery, J. & Penninckx, F. Aggregated colon cancer cells have a higher metastatic efficiency in the liver compared with nonaggregated cells: an experimental study. J. Surg. Res. 112, 31–37 (2003).
Knisely, W. H. & Mahaley, M. S. Jr. Relationship between size and distribution of spontaneous metastases and three sizes of intravenously injected particles of VX2 carcinoma. Cancer Res. 18, 900–905 (1958).
Thompson, S. C. The colony forming efficiency of single cells and cell aggregates from a spontaneous mouse mammary tumour using the lung colony assay. Br. J. Cancer 30, 332–336 (1974).
Edelman, G. M., Gallin, W. J., Delouvée, A., Cunningham, B. A. & Thiery, J. P. Early epochal maps of two different cell adhesion molecules. Proc. Natl Acad. Sci. USA 80, 4384–4388 (1983).
Kim, K. K. et al. Alveolar epithelial cell mesenchymal transition develops in vivo during pulmonary fibrosis and is regulated by the extracellular matrix. Proc. Natl Acad. Sci. USA 103, 13180–13185 (2006).
Kalluri, R. & Weinberg, R. A. The basics of epithelial–mesenchymal transition. J. Clin. Invest. 119, 1420–1428 (2009).
Kalluri, R. EMT: when epithelial cells decide to become mesenchymal-like cells. J. Clin. Invest. 119, 1417–1419 (2009).
Soltermann, A. et al. Prognostic significance of epithelial–mesenchymal and mesenchymal–epithelial transition protein expression in non-small cell lung cancer. Clin. Cancer Res. 14, 7430–7437 (2008).
Bellovin, D. I. et al. Reciprocal regulation of RhoA and RhoC characterizes the EMT and identifies RhoC as a prognostic marker of colon carcinoma. Oncogene 25, 6959–6967 (2006).
Gjerdrum, C. et al. Axl is an essential epithelial-to-mesenchymal transition-induced regulator of breast cancer metastasis and patient survival. Proc. Natl Acad. Sci. USA 107, 1124–1129 (2010).
Otsuki, S. et al. Vimentin expression is associated with decreased survival in gastric cancer. Oncol. Rep. 25, 1235–1242 (2011).
Lee, K. W. et al. Twist1 is an independent prognostic factor of esophageal squamous cell carcinoma and associated with its epithelial-mesenchymal transition. Ann. Surg. Oncol. 19, 326–335 (2012).
Byers, L. A. et al. An epithelial–mesenchymal transition gene signature predicts resistance to EGFR and PI3K inhibitors and identifies Axl as a therapeutic target for overcoming EGFR inhibitor resistance. Clin. Cancer Res. 19, 279–290 (2013).
Kurokawa, M., Ise, N., Omi, K., Goishi, K. & Higashiyama, S. Cisplatin influences acquisition of resistance to molecular-targeted agents through epithelial-mesenchymal transition-like changes. Cancer Sci. 104, 904–911 (2013).
Yauch, R. L. et al. Epithelial versus mesenchymal phenotype determines in vitro sensitivity and predicts clinical activity of erlotinib in lung cancer patients. Clin. Cancer Res. 11, 8686–8698 (2005).
Lim, S. et al. SNAI1-mediated epithelial-mesenchymal transition confers chemoresistance and cellular plasticity by regulating genes involved in cell death and stem cell maintenance. PLoS ONE 8, e66558 (2013).
Brabletz, T. et al. Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc. Natl Acad. Sci. USA 98, 10356–10361 (2001).
Stark, K., Vainio, S., Vassileva, G. & McMahon, A. P. Epithelial transformation of metanephric mesenchyme in the developing kidney regulated by Wnt-4. Nature 372, 679–683 (1994).
Hou, J. M. et al. Circulating tumor cells as a window on metastasis biology in lung cancer. Am. J. Pathol. 178, 989–996 (2011).
Lecharpentier, A. et al. Detection of circulating tumour cells with a hybrid (epithelial/mesenchymal) phenotype in patients with metastatic non-small cell lung cancer. Br. J. Cancer 105, 1338–1341 (2011).
Kallergi, G. et al. Epithelial to mesenchymal transition markers expressed in circulating tumour cells of early and metastatic breast cancer patients. Breast Cancer Res. 13, R59 (2011).
Armstrong, A. J. et al. Circulating tumor cells from patients with advanced prostate and breast cancer display both epithelial and mesenchymal markers. Mol. Cancer Res. 9, 997–1007 (2011).
Balasubramanian, P. et al. Multiparameter analysis, including EMT markers, on negatively enriched blood samples from patients with squamous cell carcinoma of the head and neck. PLoS ONE 7, e42048 (2012).
Yu, M. et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science 339, 580–584 (2013).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Singh, S. K. et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828 (2003).
Ricci-Vitiani, L. et al. Identification and expansion of human colon-cancer-initiating cells. Nature 445, 111–115 (2007).
Chan, K. S. et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc. Natl Acad. Sci. USA 106, 14016–14021 (2009).
Stewart, J. M. et al. Phenotypic heterogeneity and instability of human ovarian tumor-initiating cells. Proc. Natl Acad. Sci. USA 108, 6468–6473 (2011).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Mani, S. A. et al. The epithelial–mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 (2008).
Grimshaw, M. J. et al. Mammosphere culture of metastatic breast cancer cells enriches for tumorigenic breast cancer cells. Breast Cancer Res. 10, R52 (2008).
Fidler, I. J. Metastasis: quantitative analysis of distribution and fate of tumor embolilabeled with 125 I-5-iodo-2′-deoxyuridine. J. Natl Cancer Inst. 45, 773–782 (1970).
Luzzi, K. J. et al. Multistep nature of metastatic inefficiency: dormancy of solitary cells after successful extravasation and limited survival of early micrometastases. Am. J. Pathol. 153, 865–873 (1998).
Chambers, A. F., Groom, A. C. & MacDonald, I. C. Dissemination and growth of cancer cells in metastatic sites. Nat. Rev. Cancer 2, 563–572 (2002).
Fidler, I. J. The pathogenesis of cancer metastasis: the 'seed and soil' hypothesis revisited. Nat. Rev. Cancer 3, 453–458 (2003).
Chaffer, C. L. & Weinberg, R. A. A perspective on cancer cell metastasis. Science 331, 1559–1564 (2011).
Zhao, Q. et al. Interaction between circulating galectin-3 and cancer-associated MUC1 enhances tumour cell homotypic aggregation and prevents anoikis. Mol. Cancer 9, 154 (2010).
Fidler, I. J. Immune stimulation-inhibition of experimental cancer metastasis. Cancer Res. 34, 491–498 (1974).
Ludatscher, R. M., Luse, S. A. & Suntzeff, V. An electron microscopic study of pulmonary tumor emboli from transplantable Morris hepatoma 5123. Cancer Res. 27, 1939–1952 (1967).
Sugino, T. et al. An invasion-independent pathway of blood-borne metastasis: a new murine mammary tumor model. Am. J. Pathol. 160, 1973–1980 (2002).
Küsters, B. et al. Micronodular transformation as a novel mechanism of VEGF-A-induced metastasis. Oncogene 26, 5808–5815 (2007).
Laübli, H., Stevenson, J. L., Varki, A., Varki, N. M. & Borsig, L. L-Selectin facilitation of metastasis involves temporal induction of Fut7-dependent ligands at sites of tumor cell arrest. Cancer Res. 66, 1536–1542 (2006).
Gasic, G. J., Gasic, T. B., Galanti, N., Johnson, T. & Murphy, S. Platelet-tumor-cell interactions in mice. The role of platelets in the spread of malignant disease. Int. J. Cancer 11, 704–718 (1973).
Borsig, L., Wong, R., Hynes, R. O., Varki, N. M. & Varki, A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl Acad. Sci. USA 99, 2193–2198 (2002).
Upreti, M. et al. Tumor-endothelial cell three-dimensional spheroids: new aspects to enhance radiation and drug therapeutics. Transl. Oncol. 4, 365–376 (2011).
Fidler, I. J. & Talmadge, J. E. Evidence that intravenously derived murine pulmonary melanoma metastases can originate from the expansion of a single tumor cell. Cancer Res. 46, 5167–5171 (1986).
Talmadge, J. E., Wolman, S. R. & Fidler, I. J. Evidence for the clonal origin of spontaneous metastases. Science 217, 361–363 (1982).
Krebs, M. G. et al. Analysis of circulating tumor cells in patients with non-small cell lung cancer using epithelial marker-dependent and -independent approaches. J. Thorac. Oncol. 7, 306–315 (2012).
Tomlinson, J. S., Alpaugh, M. L. & Barsky, S. H. An intact overexpressed E-cadherin/α, β-catenin axis characterizes the lymphovascular emboli of inflammatory breast carcinoma. Cancer Res. 61, 5231–5241 (2001).
Kleer, C. G., van Golen, K. L., Braun, T. & Merajver, S. D. Persistent E-cadherin expression in inflammatory breast cancer. Mod. Pathol. 14, 458–464 (2001).
Sugino, T. et al. Morphological evidence for an invasion-independent metastasis pathway exists in multiple human cancers. BMC Med. 2, 9 (2004).
De Giorgi, U. et al. Circulating tumor cells and bone metastases as detected by FDG-PET/CT in patients with metastatic breast cancer. Ann. Oncol. 21, 33–39 (2010).
Cho, E. H. et al. Characterization of circulating tumor cell aggregates identified in patients with epithelial tumors. Phys. Biol. 9, 016001 (2012).
Griffiths, J. D., McKinna, J. A., Rowbotham, H. D., Tsolakidis, P. & Salsbury, A. J. Carcinoma of the colon and rectum: circulating malignant cells and five-year survival. Cancer 31, 226–236 (1973).
Hofman, V. et al. Preoperative circulating tumor cell detection using the isolation by size of epithelial tumor cell method for patients with lung cancer is a new prognostic biomarker. Clin. Cancer Res. 17, 827–835 (2011).
Kats-Ugurlu, G. et al. Circulating tumour tissue fragments in patients with pulmonary metastasis of clear cell renal cell carcinoma. J. Pathol. 219, 287–293 (2009).
Glaves, D., Huben, R. P. & Weiss, L. Haematogenous dissemination of cells from human renal adenocarcinomas. Br. J. Cancer 57, 32–35 (1988).
Brandt, B. et al. Isolation of prostate-derived single cells and cell clusters from human peripheral blood. Cancer Res. 56, 4556–4561 (1996).
Molnar, B., Ladanyi, A., Tanko, L., Sréter, L. & Tulassay, Z. Circulating tumor cell clusters in the peripheral blood of colorectal cancer patients. Clin. Cancer Res. 7, 4080–4085 (2001).
Wang, Z. P. et al. Identification and characterization of circulating prostate carcinoma cells. Cancer 88, 2787–2795 (2000).
Krebs, M. G., Hou, J.-M., Ward, T. H., Blackhall, F. H. & Dive, C. Circulating tumour cells: their utility in cancer management and predicting outcomes. Ther. Adv. Med. Oncol. 2, 351–365 (2010).
Gorges, T. M. & Pantel, K. Circulating tumor cells as therapy-related biomarkers in cancer patients. Cancer Immunol. Immunother. 62, 931–939 (2013).
Stott, S. L. et al. Isolation and characterization of circulating tumor cells from patients with localized and metastatic prostate cancer. Sci. Transl. Med. 2, 25ra23 (2010).
Hiltermann, T. J. et al. Circulating tumor cells in small-cell lung cancer: a predictive and prognostic factor. Ann. Oncol. 23, 2937–2942 (2012).
Naito, T. et al. Prognostic impact of circulating tumor cells in patients with small cell lung cancer. J. Thorac. Oncol. 7, 512–519 (2012).
Hayes, D. F. et al. Circulating tumor cells at each follow-up time point during therapy of metastatic breast cancer patients predict progression-free and overall survival. Clin. Cancer Res. 12, 4218–4224 (2006).
Riethdorf, S. et al. Detection and HER2 expression of circulating tumor cells: prospective monitoring in breast cancer patients treated in the neoadjuvant GeparQuattro trial. Clin. Cancer Res. 16, 2634–2645 (2010).
de Bono, J. S. et al. Potential applications for circulating tumor cells expressing the insulin-like growth factor-I receptor. Clin. Cancer Res. 13, 3611–3616 (2007).
Liu, Z. et al. Eradication of EGFR-positive circulating tumor cells and objective tumor response with lapatinib and capecitabine. Cancer Biol. Ther. 10, 860–864 (2010).
Evans, W. K. et al. Etoposide (VP-16) and cisplatin: an effective treatment for relapse in small-cell lung cancer. J. Clin. Oncol. 3, 65–71 (1985).
Bidard, F. C. et al. Clinical application of circulating tumor cells in breast cancer: overview of the current interventional trials. Cancer Metastasis Rev. 32, 179–188 (2013).
Swennenhuis, J. F., Tibbe, A. G., Levink, R., Sipkema, R. C. & Terstappen, L. W. Characterization of circulating tumor cells by fluorescence in situ hybridization. Cytometry A 75, 520–527 (2009).
Rossi, E. et al. M30 neoepitope expression in epithelial cancer: quantification of apoptosis in circulating tumor cells by CellSearch analysis. Clin. Cancer Res. 16, 5233–5243 (2010).
Ang, J. E., Kaye, S. & Banerji, U. Tissue-based approaches to study pharmacodynamic endpoints in early phase oncology clinical trials. Curr. Drug Targets 13, 1525–1534 (2012).
Wang, L. H. et al. Monitoring drug-induced gammaH2AX as a pharmacodynamic biomarker in individual circulating tumor cells. Clin. Cancer Res. 16, 1073–1084 (2010).
Kallergi, G. et al. Phosphorylated EGFR and PI3K/Akt signaling kinases are expressed in circulating tumor cells of breast cancer patients. Breast Cancer Res. 10, R80 (2008).
Flores, L. M. et al. Improving the yield of circulating tumour cells facilitates molecular characterisation and recognition of discordant HER2 amplification in breast cancer. Br. J. Cancer 102, 1495–1502 (2010).
Kallergi, G. et al. Hypoxia-inducible factor-1α and vascular endothelial growth factor expression in circulating tumor cells of breast cancer patients. Breast Cancer Res. 11, R84 (2009).
Camidge, D. R. et al. Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study. Lancet Oncol. 13, 1011–1019 (2012).
Ilie, M. et al. ALK-gene rearrangement: a comparative analysis on circulating tumour cells and tumour tissue from patients with lung adenocarcinoma. Ann. Oncol. 23, 2907–2913 (2012).
Hayes, D. F. et al. Monitoring expression of HER-2 on circulating epithelial cells in patients with advanced breast cancer. Int. J. Oncol. 21, 1111–1117 (2002).
Jiang, Y., Palma, J. F., Agus, D. B., Wang, Y. & Gross, M. E. Detection of androgen receptor mutations in circulating tumor cells in castration-resistant prostate cancer. Clin. Chem. 56, 1492–1495 (2010).
Leversha, M. A. et al. Fluorescence in situ hybridization analysis of circulating tumor cells in metastatic prostate cancer. Clin. Cancer Res. 15, 2091–2097 (2009).
Liu, Y. et al. Circulating tumor cells in HER2-positive metastatic breast cancer patients: a valuable prognostic and predictive biomarker. BMC Cancer 13, 202 (2013).
Crowley, E., Di Nicolantonio, F., Loupakis, F. & Bardelli, A. Liquid biopsy: monitoring cancer-genetics in the blood. Nat. Rev. Clin. Oncol. 10, 472–484 (2013).
De Mattos-Arruda, L. et al. Circulating tumour cells and cell-free DNA as tools for managing breast cancer. Nat. Rev. Clin. Oncol. 10, 377–389 (2013).
Dawson, S. J. et al. Analysis of circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 368, 1199–1209 (2013).
Cristofanilli, M. & Fortina, P. Circulating tumor DNA to monitor metastatic breast cancer. N. Engl. J. Med. 369, 93–94 (2013).
Taniguchi, K. et al. Quantitative detection of EGFR mutations in circulating tumor DNA derived from lung adenocarcinomas. Clin. Cancer Res. 17, 7808–7815 (2011).
Murtaza, M. et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature 497, 108–112 (2013).
Hofman, P. et al. Immunohistochemistry to identify EGFR mutations or ALK rearrangements in patients with lung adenocarcinoma. Ann. Oncol. 23, 1738–1743 (2012).
Ni, X. et al. Reproducible copy number variation patterns among single circulating tumor cells of lung cancer patients. Proc. Natl Acad. Sci. USA 110, 21083–21088 (2013).
Acknowledgements
The authors thank Cancer Research UK for research support via core funding to the Cancer Research UK Manchester Institute (C5659/A12328). R. L. Metcalf and L. Carter are supported by an unrestricted educational grant from Cancer Research UK and AstraZeneca.
Author information
Authors and Affiliations
Contributions
M. G. Krebs, R. L. Metcalf and L. Carter researched the data for the article, and discussed its content with C. Dive and F. H. Blackhall. All authors wrote the manuscript and edited it before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Krebs, M., Metcalf, R., Carter, L. et al. Molecular analysis of circulating tumour cells—biology and biomarkers. Nat Rev Clin Oncol 11, 129–144 (2014). https://doi.org/10.1038/nrclinonc.2013.253
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2013.253
This article is cited by
-
Resistance to BRAF inhibition explored through single circulating tumour cell molecular profiling in BRAF-mutant non-small-cell lung cancer
British Journal of Cancer (2024)
-
Liquid biopsy at the frontier in renal cell carcinoma: recent analysis of techniques and clinical application
Molecular Cancer (2023)
-
Clinical application and detection techniques of liquid biopsy in gastric cancer
Molecular Cancer (2023)
-
Drug-tolerant persister cells in cancer: the cutting edges and future directions
Nature Reviews Clinical Oncology (2023)
-
Hydrogel-based technologies in liquid biopsy for the detection of circulating clinical markers: challenges and prospects
Analytical and Bioanalytical Chemistry (2023)