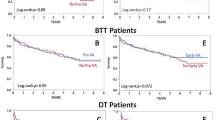
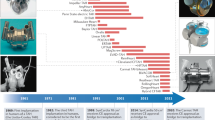
Abstract
Despite major advances in the treatment of heart failure over the past 2 decades improving the natural history of this condition, heart failure continues to be a major source of morbidity and mortality. Although availability of donor hearts for transplantation has declined over the past several years, innovations in ventricular assist device (VAD) technology has provided an alternative therapeutic option for patients with advanced heart failure. Initiated as a mechanical option to 'bridge' critically ill patients awaiting transplantation, VADs are being increasingly deployed as 'destination' devices to provide long-term support. With technical advances resulting in improved mechanical reliability, reduced postoperative morbidity and greater likelihood of patient acceptance, there is interest in expanding the applicability for destination VAD treatment beyond the current indication of severely ill patients who are not candidates for transplant. This Review examines the newer, third-generation VADs for mechanical cardiac support. These devices are at various stages of development and clinical investigation. One or more of these newer devices is likely to emerge as an important development in the treatment of patients with advanced heart failure.
Key Points
-
Mechanical cardiac support is a rapidly evolving field
-
Several new technologies are at various stages of development
-
Initial results of newer generation ventricular assist devices show encouraging results towards improved device durability and clinical outcomes
-
With these improved technologies, the potential exists for expanding the indication of ventricular assist devices, a result that will require additional clinical trials
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Rosamond, W. et al. Heart disease and stroke statistics: 2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 117, e25–e146 (2008).
Allen, L. A. et al. High mortality without ESCAPE: the registry of heart failure patients receiving pulmonary artery catheters without randomization. J. Card. Fail. 14, 661–669 (2008).
Mancini, D. & Burkhoff, D. Mechanical device-based methods of managing and treating heart failure Circulation 112, 438–448 (2005).
Marx, S. O. et al. PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101, 365–376 (2000).
McCarthy, P. M. et al. Hemodynamic and physiologic changes during support with an implantable left ventricular assist device. J. Thorac. Cardiovasc. Surg. 109, 409–417 (1995).
Klotz, S. et al. Left ventricular assist device support normalizes left and right ventricular beta-adrenergic pathway properties. J. Am. Coll. Cardiol. 45, 668–676 (2005).
Kirklin, J. K. et al. INTERMACS database for durable devices for circulatory support: first annual report. J. Heart Lung Transplant. 27, 1065–1072 (2008).
Wilson, S. R., Mudge, G. H. Jr, Stewart, G. C. & Givertz, M. M. Evaluation for a ventricular assist device: selecting the appropriate candidate. Circulation 119, 2225–2232 (2009).
Frazier, O. H. et al. Improved mortality and rehabilitation of transplant candidates treated with a long-term implantable left ventricular assist system. Ann. Surg. 222, 327–338 (1995).
Patlolla, V., Patten, R. D., DeNofrio, D., Konstam, M. A. & Krishnamani, R. The effect of ventricular assist devices on post-transplant mortality: analysis of United Network for Organ Sharing Network Registry. J. Am. Coll. Cardiol. 53, 264–271 (2009).
Rose, E. A. et al. Long-term use of a left ventricular assist device for end stage heart failure. N. Engl. J. Med. 20, 1435–1443 (2001).
Park, S. J. et al. Left ventricular assist devices as destination therapy: a new look at survival. J. Thorac. Cardiovasc. Surg. 129, 9–17 (2005).
Long, J. W. et al. Improving outcomes with long-term “destination” therapy using left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 135, 1353–1361 (2008).
Leitz, K. et al. Outcomes of left ventricular assist device implantation as destination therapy in the post-REMATCH Era. Implications for patient selection. Circulation 116, 497–505 (2007).
National Heart Lung and Blood Institute, National Institutes of Health, Division of Cardiovascular Diseases Strategic Plan Goal 2.4d [online], (2009).
National Heart Lung and Blood Institute, National Institutes of Health, NHLBI Working Group Clinical Use of Ventricular Assist Devices [online], (2009).
Hutchinson, J. et al. Cost-effectiveness of left ventricular-assist devices in end-stage heart failure. Expert Rev. Cardiovasc. Ther. 6, 175–185 (2008).
Moskowitz, A. J., Rose, E. A. & Gelijns, A. C. The cost of long-term LVAD implantation. Ann. Thorac. Surg. 71 (Suppl. 3), S195–S198 (2001).
Sharples, L. D. et al. Cost-effectiveness of ventricular assist device use in the United Kingdom: results from the evaluation of ventricular assist device programme in the UK (EVAD-UK). J. Heart Lung Transplant. 25, 1336–1343 (2006).
Pagani, F. D. et al. Improved mechanical reliability of the HeartMate XVE left ventricular assist system. Ann. Thorac. Surg. 82, 1413–1419 (2006).
Taylor, D. O. et al. Registry of the International Society for Heart and Lung Transplantation: twenty-fourth official adult heart transplant report—2007. J. Heart Lung Transplant. 26, 769–781 (2008).
Klovaite, J., Gustafsson, F., Mortensen, S., Sander, K. & Nielsen, L. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II). J. Am. Coll. Cardiol. 5 3, 2162–2167 (2009).
Crow, S. et al. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J. Thorac. Cardiovasc. Surg. 137, 208–215 (2009).
Grasso, M., Fenkel, J., Sorensen, E. & Feller, E. Gastrointestinal bleeding from arteriovenous malformations in recipients of left ventricular assist devices. J. Card. Fail. 13, S115 (2007).
Miller, L. W. et al. Use of a continuous-flow device in patients awaiting heart transplantation. N. Engl. J. Med. 357, 885–896 (2007).
Pagani, F. D. et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J. Am. Coll. Cardiol. 54, 312–321 (2009).
Pagani, F. D. Continuous-flow rotary left ventricular assist devices with '3rd generation' design. Semin. Thorac. Cardiovasc. Surg. 20, 255–263 (2008).
Esmore, D. et al. Initial clinical experience with the ventrassist left ventricular assist device: the pilot trial. J. Heart Lung Transplant. 27, 479–485 (2008).
Esmore, D. et al. VentrAssistTM left ventricular assist device: clinical trial results and clinical development plan update. Eur. J. Cardiothorac. Surg. 32, 735–744 (2007).
Boyle, A. J. et al. The ventrassist US pivotal bridge to cardiac transplantation trial. Presented at the 58th annual meeting of the American College of Cardiology, March 30, 2009.
Slaughter, M. S. et al. HeartWare miniature axial-flow ventricular assist device-design and initial feasibility test. Texas Heart Inst. J. 36, 12–16 (2009). Erratum in: Texas Heart Inst. J. 36, 186 (2009).
Tuzun, E. et al. In vivo evaluation of the Heart-Ware centrifugal ventricular assist device. Texas Heart Inst. J. 34, 406–411 (2007).
Nishinaka, T. et al. The DuraHeart VAD, a magnetically levitated centrifugal pump—The University of Vienna bridge to transplant experience. Circulation 70, 1421–1425 (2006).
Morshuis, M. et al. European experience of DuraHeart magnetically levitated centrifugal left ventricular assist system. Eur. J. Cardiothorac. Surg. 35, 1020–1027 (2009).
Pitsis, A. A. et al. First human implantation of a new rotary blood pump: design of the clinical feasibility study. Hellenic J. Cardiol. 47, 368–376 (2006).
Schmid, C. et al. Influence of inflow cannula length in axial-flow pumps on neurologic adverse event rate: results from a multicenter analysis. J. Heart Lung Transplant. 27, 253–260 (2008).
Schmid, C. et al. First clinical experience with the Incor left ventricular assist device. J. Heart Lung Transplant. 24, 1188–1194 (2005).
Hetzer, R. et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur. J. Cardiothorac. Surg. 25, 964–970 (2004).
Meyns, B. et al. Proof of concept: hemodynamic response to long-term partial ventricular support with the synergy pocket micro-pump. J. Am. Coll. Cardiol. 54, 79–86 (2009).
Meyns, B. et al. First human use of partial left ventricular heart support with the Circulite synergy micro-pump as a bridge to cardiac transplantation. Eur. Heart J. 29, 2582 (2008).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
R. Krishnamami and D. DeNofrio have received grants or research support from HeartWare and Thoratec. M. A. Konstam has received research support from HeartWare.
Rights and permissions
About this article
Cite this article
Krishnamani, R., DeNofrio, D. & Konstam, M. Emerging ventricular assist devices for long-term cardiac support. Nat Rev Cardiol 7, 71–76 (2010). https://doi.org/10.1038/nrcardio.2009.222
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2009.222
This article is cited by
-
An Accelerated Thrombosis Model for Computational Fluid Dynamics Simulations in Rotary Blood Pumps
Cardiovascular Engineering and Technology (2022)
-
Interdisciplinarity in New Product Development in an Indian MedTech Perspective: Gap and the Solution
Health and Technology (2019)
-
High fidelity computational simulation of thrombus formation in Thoratec HeartMate II continuous flow ventricular assist device
Scientific Reports (2016)
-
Cardiogenic shock in ACS. Part 2: role of mechanical circulatory support
Nature Reviews Cardiology (2012)
-
An implantable Fabry-Pérot pressure sensor fabricated on left ventricular assist device for heart failure
Biomedical Microdevices (2012)