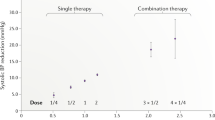
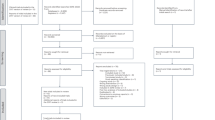
Abstract
The pharmaceutical development of a cardiovascular polypill presents several unique challenges. The selection of the type and number of active drugs to be incorporated requires important consideration of clinical, pharmaceutical and commercial issues, and the final decision with regard to the polypill's components depends on how these issues are prioritized. Once the drug combination has been chosen, developers must determine which pharmaceutical formulation should be used. The most appropriate method of drug delivery can vary markedly and depends on the characteristics of the drugs to be combined. Finally, careful consideration of how to gather the type of information required by regulatory agencies before a particular polypill can be approved for use in the general population is crucial. Although the association of multiple, active ingredients in a single dosage form would represent a major step forward in the prevention of cardiovascular conditions, a careful evaluation of all the above-mentioned variables and a well thought-out development plan is mandatory to maximize the chances of success.
Key Points
-
Pharmaceutical development of a cardiovascular polypill is a difficult task that involves consideration of formulation, analytical, intellectual property, clinical and regulatory issues
-
An association of two or three active ingredients in a cardiovascular polypill probably represents the most acceptable compromise for these issues
-
The simplest and most-straightforward approach to polypill formulation is to develop a unique mixture of all the active ingredients; however, other approaches that physically separate the individual components are available for ingredients with incompatible properties
-
Clinical development plans might vary depending on the health conditions to be prevented and the intended clinical use of the polypill
-
Discussion with regulatory agencies before development starts is highly advisable
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Wald NJ and Law MR (2003) A strategy to reduce cardiovascular disease by more than 80%. BMJ 326: 1419–1423
Law MR et al. (2003) Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 326: 1423–1427
Law MR et al. (2003) Value of low dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ 326: 1427–1434
Scheen AJ et al. (2003) Prevention of cardiovascular diseases using a combined pharmacological approach: is there any place for a “polypill”? [French] Rev Med Liege 58: 527–533
Colvin HP (2004) Polypill debate continues: people will always be sceptical. BMJ 328: 289
Willis J (2004) Polypill debate continues: concept is a fascinating thought experiment. BMJ 328: 289
Franco OH et al. (2004) The polymeal: a more natural, safer, and probably tastier (than the polypill) strategy to reduce cardiovascular disease by more than 75%. BMJ 329: 1447–1450
Wise J (2005) Polypill holds promise for people with chronic disease. Bull World Health Organ 83: 885–887
Fahey T et al. (2005) The polypill and cardiovascular disease. BMJ 330: 1035–1036
Combination Pharmacotherapy and Public Health Research Working Group (2005) Combination pharmacotherapy for cardiovascular disease. Ann Intern Med 143: 593–599
Rosenthal T and Gavras I (2006) Fixed-drug combinations as first-line treatment for hypertension. Prog Cardiovasc Dis 48: 416–425
Franco OH et al. (2006) The polypill: at what price would it become cost effective. J Epidemiol Community Health 60: 213–217
Costantino G et al. (2007) Prevention of cardiovascular disease with a polypill. Lancet 369: 185–186
Jamieson MJ and Naghavi M (2007) Multi-constituent cardiovascular pills (MCCP)—challenges and promises of population-based prophylactic drug therapy for prevention of heart attack. Curr Pharm Des 13: 1069–1076
Sleight P et al. (2006) Benefits, challenges, and registrability of the polypill. Eur Heart J 27: 1651–1656
Fuster V and Sanz G (2007) A polypill for secondary prevention: time to move from intellectual debate to action. Nat Clin Pract Cardiovasc Med 4: 173
Hennekens CH (2008) Fixed-dose combination therapy with statins: strengths, limitations and clinical and regulatory considerations. Am J Cardiovasc Drugs 8: 155–160
Nainggolan L (online 18 January 2007) Clinical trials of polypills begin. [http://www.theheart.org/article/765781.do] (accessed 4 November 2008)
World Heart Federation (online 19 May 2008) Polypill reaches clinical testing phase. [http://www.world-heart-federation.org/press/press-releases/news-details/article/polypill-reaches-clinical-testing-phase-1/] (accessed 4 November 2008)
Frishman WH and Zuckerman AL (2004) Amlodipine/atorvastatin: the first cross risk factor for polypill for the prevention and treatment of cardiovascular disease. Expert Rev Cardiovasc Ther 3: 675–681
The Scandinavian Simvastatin Survival Study Group (1994) Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 344: 1383–1389
Gotto AM Jr (2005) Review of primary and secondary prevention trials with lovastatin, pravastatin and simvastatin. Am J Cardiol 96: 34F–38F
Maroo BP et al. (2008) Secondary prevention of coronary heart disease in elderly patients following myocardial infarction: are all HMG-CoA reductase inhibitors alike? Drugs Aging 25: 649–664
Efthimiadis A (2008) Rosuvastatin and cardiovascular disease: did the strongest statin hold the initial promises? Angiology 59 (Suppl): 62S–64S
Ellison DK et al. (1993) Simvastatin. Profile of Drug Substances, Excipients and Related Methodology 22: 359–388
Schachter M (2005) Chemical, pharmacokinetic and pharmacodynamic properties of statins: an update. Fundam Clin Pharmacol 19: 117–125
Nafadi M (2000) Compatibility study between simvastatin and certain excipients. Bulletin of the Faculty of Pharmacy (Cairo University) 38: 21–32
Pasha MK et al. (2006) Analysis of 5 HMG-CoA reductase inhibitors—atorvastatin, lovastatin, pravastatin, rosuvastatin and simvastatin: pharmacological, pharmacokinetic and analytical overview and development of a new method for use in pharmaceutical formulations analysis and in vitro metabolism studies. Biomed Chomatogr 20: 282–293
Zyer I et al. (1999) Compatibility study of pravastatin sodium and pharmaceutical excipients. Res Dev Div 50: 300–301
Florey K (1979) Aspirin. Profiles of Drug Substances, Excipients and Related Methodology 8: 1–46
Ahlneck C and Waltersson J (1986) Factorial designs in pharmaceutical preformulation studies. II. Studies on drug stability and compatibility in the solid state. Acta Pharm Suec 23: 139–150
Carstensen J and Attarchi F (1988) Decomposition of aspirin in the solid state in the presence of limited amounts of moisture II: Kinetics and salting-in of aspirin in aqueous acetic acid solutions. J Pharm Sci 77: 314–317
Cavezzan J et al. (2005) And if you'll take an aspirin tablet? The development of pharmaceutical tablets: an example of multidisciplinary research [French]. Actualite Chimique 287: 35–41
Ceschel GC et al. (2003) Degradation of components in drug formulations: a comparison between HPLC and DSC methods. J Pharm Biomed Anal 32: 1067–1072
Wissing S et al. (2000) An investigation into the use of stepwise isothermal high sensitivity DSC as a means of detecting drug-excipient incompatibility. Int J Pharm 199: 141–150
Jacob M et al. (1979) [Testings of compatibility between diverse active compounds and excipients for tablets (author's transl)] [French]. J Pharm Belg 34: 96–98
European Medicines Agency and Committee for Medicinal Products for Human Use (CHMP) (2005) Questions and answers document on the clinical development of fixed combinations of drugs belonging to different therapeutic classes in the field of cardiovascular treatment and prevention. [http://www.emea.europa.eu] (accessed 4 November 2008)
European Medicines Agency and Committee for Medicinal Products for Human Use (CHMP) (2008) Guideline on fixed combination medicinal products. [http://www.emea.europa.eu] (accessed 4 November 2008)
Food and Drug Administration (FDA) (2004) Guidance for industry and FDA current good manufacturing practice for combination products. [http://www.fda.gov/oc/combination/OCLove1dft.html] (accessed 4 November 2008)
Chapman RH et al. (2003) Adherence with concomitant antihypertensive and lipid lowering therapy. Circulation 108: IV757
Schwartz JS et al. (2003) Adherence to chronic therapy among patients treated for hypertension, dyslipidemia or both. J Am Coll Cardiol 41: 526
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
A Guglietta and M Guerrero are patent holders/applicants and stock holders/directors of Grupo Ferrer Internacional, Barcelona, Spain.
Rights and permissions
About this article
Cite this article
Guglietta, A., Guerrero, M. Issues to consider in the pharmaceutical development of a cardiovascular polypill. Nat Rev Cardiol 6, 112–119 (2009). https://doi.org/10.1038/ncpcardio1424
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ncpcardio1424
This article is cited by
-
Could patents interfere with the development of a cardiovascular polypill?
Journal of Translational Medicine (2016)
-
Bisoprolol and Bisoprolol-Valsartan Compatibility Studied by Differential Scanning Calorimetry, Nuclear Magnetic Resonance and X-Ray Powder Diffractometry
Pharmaceutical Research (2015)
-
Assessing Patterns of Use of Cardio-Protective Polypill Component Medicines in Australian Women
Drugs & Aging (2013)
-
Long-Term Efficacy and Tolerability of a Fixed-Dose Combination of Antihypertensive Agents
Clinical Drug Investigation (2011)
-
Beyond Cholesterol in the Primary Prevention of Cardiovascular Disease: Is The Polypill the Answer?
Current Cardiovascular Risk Reports (2011)