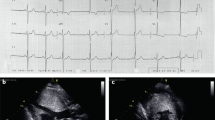
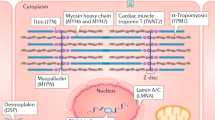
Key Points
-
Early description of cardiomyopathies originated from severe, paradigmatic case series that described worst-case scenarios
-
The entire range of cardiomyopathies has subsequently emerged, including mild phenotypes that might overlap with normal variation
-
Current screening policies have the potential to identify affected individuals at very early stages, leading to effective prevention of cardiomyopathy-related complications, including sudden cardiac death, but also increase the risk of overdiagnosing healthy individuals
-
A systematic approach is necessary in patients with mild phenotypes suggestive of, but not definitely diagnostic for, cardiomyopathies
-
We propose that at least one of five major criteria should be fulfilled before a diagnosis of cardiomyopathy is made
Abstract
Genetic cardiomyopathies are complex diseases with heterogeneous clinical presentation and phenotypes. Early descriptions of cardiomyopathies originated from case studies involving individuals with severe, paradigmatic presentation, which provided insight into the worst-case scenarios of these conditions. With time, improved diagnostic sensitivity and awareness of cardiomyopathies has uncovered a more heterogeneous disease spectrum, including mild phenotypes overlapping with physiological variation. This diagnostic 'grey area' poses important dilemmas, particularly in athletes. Current screening policies have the potential to identify affected individuals at very early stages, leading to effective prevention of cardiomyopathy-related complications such as sudden cardiac death. Conversely, however, some physicians actively impose diagnoses on individuals who perceive themselves to be disease-free. In addition, the high sensitivity of contemporary diagnostic techniques carries a serious risk of misinterpreting physiological variation as disease. In this Review, three of the most common and controversial areas are discussed, including left ventricular hypertrophy; left ventricular dilatation, noncompaction, and fibrosis; and arrhythmias originating from the right ventricle. A systematic and cautious approach is necessary in patients with mild phenotypes suggestive of, but not definitely diagnostic for, cardiomyopathies. Preventing the mislabelling of healthy individuals and overdiagnosis should be a priority, with the aim to combine adequate counselling and optimal protection.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Maron, B. J. et al. Contemporary definitions and classification of the cardiomyopathies: an American Heart Association Scientific Statement from the Council on Clinical Cardiology, Heart Failure and Transplantation Committee; Quality of Care and Outcomes Research and Functional Genomics and Translational Biology Interdisciplinary Working Groups; and Council on Epidemiology and Prevention. Circulation 113, 1807–1816 (2006).
Hershberger, R. E., Cowan, J., Morales, A. & Siegfried, J. D. Progress with genetic cardiomyopathies screening, counseling, and testing in dilated, hypertrophic, and arrhythmogenic right ventricular dysplasia/cardiomyopathy. Circ. Heart Fail. 2, 253–261 (2009).
Heath, I. Role of fear in overdiagnosis and overtreatment — an essay by Iona Heath. BMJ 349, g6123 (2014).
Corrado, D. et al. Trends in sudden cardiovascular death in young competitive athletes after implementation of a preparticipation screening program. JAMA 296, 1593–1601 (2006).
Maron, B. J. et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: preamble, principles, and general considerations: a scientific statement from the American Heart Association and American College of Cardiology. Circulation 132, e256–e261 (2015).
Sheikh, N. et al. Comparison of electrocardiographic criteria for the detection of cardiac abnormalities in elite black and white athletes. Circulation 129, 1637–1649 (2014).
Papadakis, M. et al. Prevalence and significance of T-wave inversions in predominantly Caucasian adolescent athletes. Eur. Heart J. 30, 1728–1735 (2009).
Magalski, A. et al. Relation of race to electrocardiographic patterns in elite American football players. J. Am. Coll. Cardiol. 51, 2250–2255 (2008).
Papadakis, M. et al. The prevalence, distribution, and clinical outcomes of electrocardiographic repolarization patterns in male athletes of African/Afro-Caribbean origin. Eur. Heart J. 32, 2304–2313 (2011).
Pelliccia, A., Maron, B. J., Spataro, A., Proschan, M. A. & Spirito, P. The upper limit of physiologic cardiac hypertrophy in highly trained elite athletes. N. Engl. J. Med. 324, 295–301 (1991).
Rawlins, J. et al. Ethnic differences in physiological cardiac adaptation to intense physical exercise in highly trained female athletes. Circulation 121, 1078–1785 (2010).
Pelliccia, A., Maron, B. J., Culasso, F., Spataro, A. & Caselli, G. Athlete's heart in women. Echocardiographic characterization of highly trained elite female athletes. JAMA 276, 211–215 (1996).
Drezner, J. A. et al. Normal electrocardiographic findings: recognising physiological adaptations in athletes. Br. J. Sports Med. 47, 125–136 (2013).
Corrado, D. et al. Recommendations for interpretation of 12-lead electrocardiogram in the athlete. Eur. Heart J. 31, 243–259 (2010).
Drezner, J. A. et al. Abnormal electrocardiographic findings in athletes: recognising changes suggestive of cardiomyopathy. Br. J. Sports Med. 47, 137–152 (2013).
Pelliccia, A. et al. Outcomes in athletes with marked ECG repolarization abnormalities. N. Engl. J. Med. 358, 152–161 (2008).
Schnell, F. et al. Recognition and significance of pathological T-wave inversions in athletes. Circulation 131, 165–173 (2015).
Caselli, S. et al. Differentiating left ventricular hypertrophy in athletes from that in patients with hypertrophic cardiomyopathy. Am. J. Cardiol. 114, 1383–1389 (2014).
Maron, B. J. Distinguishing hypertrophic cardiomyopathy from athlete's heart physiological remodelling: clinical significance, diagnostic strategies and implications for preparticipation screening. Br. J. Sports Med. 43, 649–656 (2009).
Sheikh, N. et al. Clinical profile of athletes with hypertrophic cardiomyopathy: the “grey zone” revisited. Circ. Cardiovasc. Imaging 8, e003454 (2015).
Flett, A. S. et al. Diagnosis of apical hypertrophic cardiomyopathy: T-wave inversion and relative but not absolute apical left ventricular hypertrophy. Int. J. Cardiol. 183, 143–148 (2015).
Naylor, L. H., George, K., O'Driscoll, G. & Green, D. J. The athlete's heart: a contemporary appraisal of the 'Morganroth hypothesis'. Sports Med. 38, 69–90 (2008).
Haykowsky, M. J. & Tomczak, C. R. LV hypertrophy in resistance or endurance trained athletes: the Morganroth hypothesis is obsolete, most of the time. Heart 100, 1225–1226 (2014).
Elliott, P. M. et al. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur. Heart J. 35, 2733–2779 (2014).
Ho, C. Y. et al. Assessment of diastolic function with Doppler tissue imaging to predict genotype in preclinical hypertrophic cardiomyopathy. Circulation 105, 2992–2997 (2002).
Kauer, F. et al. Diastolic abnormalities in normal phenotype hypertrophic cardiomyopathy gene carriers: a study using speckle tracking echocardiography. Echocardiography 30, 558–563 (2013).
Sharma, S. et al. Utility of cardiopulmonary exercise in the assessment of clinical determinants of functional capacity in hypertrophic cardiomyopathy. Am. J. Cardiol. 86, 162–168 (2000).
Olivotto, I. et al. Prognostic value of systemic blood pressure response during exercise in a community-based patient population with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 33, 2044–2051 (1999).
Gimeno, J. R. et al. Exercise-induced ventricular arrhythmias and risk of sudden cardiac death in patients with hypertrophic cardiomyopathy. Eur. Heart J. 30, 2599–2605 (2009).
Weiner, R. B. et al. Regression of “gray zone” exercise-induced concentric left ventricular hypertrophy during prescribed detraining. J. Am. Coll. Cardiol. 59, 1992–1994 (2012).
Leone, O. et al. 2011 consensus statement on endomyocardial biopsy from the Association for European Cardiovascular Pathology and the Society for Cardiovascular Pathology. Cardiovasc. Pathol. 21, 245–274 (2012).
Walsh, R. et al. Reassessment of Mendelian gene pathogenicity using 7,855 cardiomyopathy cases and 60,706 reference samples. Genet. Med. http://dx.doi.org/doi:10.1038/gim.2016.90 (2016).
Mogensen, J. et al. The current role of next-generation DNA sequencing in routine care of patients with hereditary cardiovascular conditions: a viewpoint paper of the European Society of Cardiology working group on myocardial and pericardial diseases and members of the European Society of Human Genetics. Eur. Heart J. 36, 1367–1370 (2015).
Bogaert, J. & Olivotto, I. MR imaging in hypertrophic cardiomyopathy: from magnet to bedside. Radiology 273, 329–348 (2014).
Olivotto, I. et al. Assessment and significance of left ventricular mass by cardiovascular magnetic resonance in hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 52, 559–566 (2008).
Ingles, J., Burns, C., Barratt, A. & Semsarian, C. Application of genetic testing in hypertrophic cardiomyopathy for preclinical disease detection. Circ. Cardiovasc. Genet. 8, 852–859 (2015).
Kwon, J. M. & Steiner, R. D. “I'm fine; I'm just waiting for my disease”: the new and growing class of presymptomatic patients. Neurology 77, 522–523 (2011).
Valente, A. M. et al. Comparison of echocardiographic and cardiac magnetic resonance imaging in hypertrophic cardiomyopathy sarcomere mutation carriers without left ventricular hypertrophy. Circ. Cardiovasc. Genet. 6, 230–237 (2013).
Maron, M. S. et al. Prevalence and clinical profile of myocardial crypts in hypertrophic cardiomyopathy. Circ. Cardiovasc. Imaging 5, 441–447 (2012).
Maron, M. S. et al. Mitral valve abnormalities identified by cardiovascular magnetic resonance represent a primary phenotypic expression of hypertrophic cardiomyopathy. Circulation 124, 40–47 (2011).
Gruner, C. et al. Significance of left ventricular apical–basal muscle bundle identified by cardiovascular magnetic resonance imaging in patients with hypertrophic cardiomyopathy. Eur. Heart J. 35, 2706–2713 (2014).
Ho, C. Y. et al. Myocardial fibrosis as an early manifestation of hypertrophic cardiomyopathy. N. Engl. J. Med. 363, 552–563 (2010).
Maron, B. J. et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 3: hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy and other cardiomyopathies, and myocarditis: a scientific statement from the American Heart Association and American College of Cardiology. J. Am. Coll. Cardiol. 66, 2362–2371 (2015).
Pelliccia, A. et al. Recommendations for competitive sports participation in athletes with cardiovascular disease. Eur. Heart J. 26, 1422–1445 (2005).
Olivotto, I., Cecchi, F., Poggesi, C. & Yacoub, M. H. Patterns of disease progression in hypertrophic cardiomyopathy: an individualized approach to clinical staging. Circ. Heart Fail. 5, 535–546 (2012).
Olivotto, I. et al. The many faces of hypertrophic cardiomyopathy: from developmental biology to clinical practice. J. Cardiovasc. Transl Res. 2, 349–367 (2009).
George, K. et al. The endurance athletes heart: acute stress and chronic adaptation. Br. J. Sports Med. 46, i29–i36 (2012).
Pelliccia, A., Culasso, F., Di Paolo, F. M. & Maron, B. J. Physiologic left ventricular cavity dilatation in elite athletes. Ann. Intern. Med. 130, 23–31 (1999).
Abernethy, W. B., Choo, J. K. & Hutter, A. M. Echocardiographic characteristics of professional football players. J. Am. Coll. Cardiol. 41, 280–284 (2003).
Abergel, E. et al. Serial left ventricular adaptations in world-class professional cyclists: implications for disease screening and follow-up. J. Am. Coll. Cardiol. 44, 144–149 (2004).
Grünig, E. et al. Frequency and phenotypes of familial dilated cardiomyopathy. J. Am. Coll. Cardiol. 31, 186–194 (1998).
Kim, J. H. et al. Significance of electrocardiographic right bundle branch block in trained athletes. Am. J. Cardiol. 107, 1083–1089 (2011).
Zaidi, A. et al. Physiological right ventricular adaptation in elite athletes of African and Afro-Caribbean origin. Circulation 127, 1783–1792 (2013).
Lehrke, S. et al. Use of cardiovascular magnetic resonance for risk stratification in chronic heart failure: prognostic value of late gadolinium enhancement in patients with non-ischaemic dilated cardiomyopathy. Heart 97, 727–732 (2011).
Chan, R. H. et al. Prognostic value of quantitative contrast-enhanced cardiovascular magnetic resonance for the evaluation of sudden death risk in patients with hypertrophic cardiomyopathy. Circulation 130, 484–495 (2014).
Whyte, G. et al. Physiological profile and predictors of cycling performance in ultra-endurance triathletes. J. Sports Med. Phys. Fitness 40, 103–109 (2000).
Duncan, A. M., Francis, D. P., Gibson, D. G. & Henein, M. Y. Limitation of exercise tolerance in chronic heart failure: distinct effects of left bundle-branch block and coronary artery disease. J. Am. Coll. Cardiol. 43, 1524–1531 (2004).
Pelliccia, A. et al. Remodeling of left ventricular hypertrophy in elite athletes after long-term deconditioning. Circulation 105, 944–949 (2002).
Caforio, A. L. et al. Current state of knowledge on aetiology, diagnosis, management, and therapy of myocarditis: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 34, 2636–2648 (2013).
Parks, S. B. et al. Lamin A/C mutation analysis in a cohort of 324 unrelated patients with idiopathic or familial dilated cardiomyopathy. Am. Heart J. 156, 161–169 (2008).
Grant, R. T. An unusual anomaly of the coronary vessels in the malformed heart of a child. Heart 13, 273–283 (1926).
Chin, T. K., Perloff, J. K., Williams, R. G., Jue, K. & Mohrmann, R. Isolated noncompaction of left ventricular myocardium. A study of eight cases. Circulation 82, 507–513 (1990).
Arbustini, E., Weidemann, F. & Hall, J. L. Left ventricular noncompaction: a distinct cardiomyopathy or a trait shared by different cardiac diseases? J. Am. Coll. Cardiol. 64, 1840–1850 (2014).
Xing, Y. et al. Genetic analysis in patients with left ventricular noncompaction and evidence for genetic heterogeneity. Mol. Genet. Metab. 88, 71–77 (2006).
Jenni, R., Oechslin, E., Schneider, J., Attenhofer Jost, C. & Kaufmann, P. A. Echocardiographic and pathoanatomical characteristics of isolated left ventricular non-compaction: a step towards classification as a distinct cardiomyopathy. Heart 86, 666–671 (2001).
Petersen, S. E. et al. Left ventricular non-compaction: insights from cardiovascular magnetic resonance imaging. J. Am. Coll. Cardiol. 46, 101–105 (2005).
Boyd, M. T., Seward, J. B., Jamil Tajik, A. A. & Edwards, W. D. Frequency and location of prominent left ventricular trabeculations at autopsy in 474 normal human hearts: implications for evaluation of mural thrombi by two-dimensional echocardiography. J. Am. Coll. Cardiol. 9, 323–326 (1987).
Kohli, S. K. et al. Diagnosis of left-ventricular non-compaction in patients with left-ventricular systolic dysfunction: time for a reappraisal of diagnostic criteria? Eur. Heart J. 29, 89–95 (2008).
Paterick, T. E. et al. Left ventricular noncompaction: a 25-year odyssey. J. Am. Soc. Echocardiogr. 25, 363–375 (2012).
Paterick, T. E. & Tajik, A. J. Left ventricular noncompaction: a diagnostically challenging cardiomyopathy. Circ. J. 76, 1556–1562 (2012).
Captur, G., Flett, A. S., Jacoby, D. L & Moon, J. C. Left ventricular non-noncompaction: the mitral valve prolapse of the 21st century? Int. J. Cardiol. 164, 3–6 (2013).
Paterick, T. E. & Tajik, A. J. Left ventricular noncompaction cardiomyopathy: lessons from the past to explain a diagnostic conundrum. J. Am. Soc. Echocardiogr. 27, 1128–1130 (2014).
Zemrak, F. et al. The relationship of left ventricular trabeculation to ventricular function and structure over a 9.5-year follow-up: the MESA study. J. Am. Coll. Cardiol. 64, 1971–1980 (2014).
Basso, C., Corrado, D., Marcus, F. I., Nava, A. & Thiene, G. Arrhythmogenic right ventricular cardiomyopathy. Lancet 373, 1289–1300 (2009).
Gaita, F. et al. Long-term follow-up of right ventricular monomorphic extrasystoles. J. Am. Coll. Cardiol. 38, 364–370 (2001).
Santangeli, P. et al. Noninvasive diagnosis of electroanatomic abnormalities in arrhythmogenic right ventricular cardiomyopathy. Circ. Arrhythm. Electrophysiol. 3, 632–638 (2010).
O'Donnell, D., Cox, D., Bourke, J., Mitchell, L. & Furniss, S. Clinical and electrophysiological differences between patients with arrhythmogenic right ventricular dysplasia and right ventricular outflow tract tachycardia. Eur. Heart J. 24, 801–810 (2003).
Ellison, K. E., Friedman, P. L., Ganz, L. I. & Stevenson, W. G. Entrainment mapping and radiofrequency catheter ablation of ventricular tachycardia in right ventricular dysplasia. J. Am. Coll. Cardiol. 32, 724–728 (1998).
Miljoen, H. et al. Electroanatomic mapping characteristics of ventricular tachycardia in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Europace 7, 516–524 (2005).
Cox, M. G. et al. Activation delay and VT parameters in arrhythmogenic right ventricular dysplasia/cardiomyopathy: toward improvement of diagnostic ECG criteria. J. Cardiovasc. Electrophysiol. 19, 775–781 (2008).
Hoffmayer, K. S. et al. Electrocardiographic comparison of ventricular arrhythmias in patients with arrhythmogenic right ventricular cardiomyopathy and right ventricular outflow tract tachycardia. J. Am. Coll. Cardiol. 58, 831–838 (2011).
Corrado, D. et al. Three-dimensional electroanatomic voltage mapping increases accuracy of diagnosing arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation 111, 3042–3050 (2005).
Quarta, G. et al. Familial evaluation in arrhythmogenic right ventricular cardiomyopathy: impact of genetics and revised task force criteria. Circulation 123, 2701–2709 (2011).
Marcus, F. I. et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia proposed modification of the task force criteria. Circulation 121, 1533–1541 (2010).
Pinto, Y. M. et al. Proposal for a revised definition of dilated cardiomyopathy, hypokinetic non-dilated cardiomyopathy, and its implications for clinical practice: a position statement of the ESC working group on myocardial and pericardial diseases. Eur. Heart J. 37, 1850–1858 (2016).
Maron, B. J. et al. Hypertrophic cardiomyopathy: present and future, with translation into contemporary cardiovascular medicine. J. Am. Coll. Cardiol. 64, 83–99 (2014).
Acknowledgements
I.O. is supported by the Italian Ministry of Health: “Left ventricular hypertrophy in aortic valve disease and hypertrophic cardiomyopathy: genetic basis, biophysical correlates and viral therapy models” (RF-2013-02356787), and “Mechanisms and treatment of coronary microvascular dysfunction in patients with genetic or secondary left ventricular hypertrophy” (NET-2011-02347173).
Author information
Authors and Affiliations
Contributions
G.Q. and L.O. researched data for the article. G.Q., M.P., P.D.D., N.M., A.I., and L.O. wrote the manuscript, and G.Q., M.P., A.G., M.S., and L.O. reviewed and edited the manuscript before submission. All the authors contributed to discussion of content.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Quarta, G., Papadakis, M., Donna, P. et al. Grey zones in cardiomyopathies: defining boundaries between genetic and iatrogenic disease. Nat Rev Cardiol 14, 102–112 (2017). https://doi.org/10.1038/nrcardio.2016.175
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2016.175
This article is cited by
-
Ventricular non-compaction review
Heart Failure Reviews (2022)
-
Auxiliary diagnostic potential of ventricle geometry and late gadolinium enhancement in left ventricular non-compaction; non-randomized case control study
BMC Cardiovascular Disorders (2017)