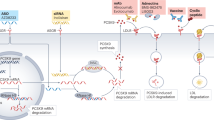
Abstract
The widespread clinical use of statins has contributed to significant reductions in the rate of cardiovascular morbidity and mortality over the past 3 decades, and statins are considered first-line therapy for the prevention and treatment of atherosclerotic vascular disease. Nevertheless, various other lipid-lowering agents can provide clinical benefit by supplementing or augmenting statin therapy in patients with severe hypercholesterolaemia or mixed dyslipidaemia, or by providing an alternative for patients who are intolerant to statins. Bile acid resins and niacin were prescribed for lipid modification for years before the introduction of the statins, and new data continue to emerge regarding their use in different patient groups and for specific conditions. Ezetimibe can be appropriate for patients whose primary lipid abnormality is an elevated LDL-cholesterol level, whereas the fibrates seem to be most beneficial in patients with low levels of HDL cholesterol and elevated triglycerides. At the end of 2012 and the beginning of 2013, the first microsomal triglyceride transfer protein inhibitor, lomitapide, and the first antisense therapy to target apolipoprotein B, mipomersen, were approved for the treatment of individuals with extremely elevated LDL-cholesterol levels caused by homozygous familial hypercholesterolaemia. Although two agents in the experimental class of cholesteryl ester transfer protein inhibitors have failed to show a benefit in clinical trials, newer drugs in this class could provide an additional strategy to address residual cardiovascular risk in patients treated with statins.
Key Points
-
Statin therapy is the mainstay of lipid-lowering treatment, but residual cardiovascular risk remains unacceptably high in patients with coronary heart disease receiving optimal statin therapy
-
Patients with statin intolerance, mixed dyslipidaemia, or an extremely elevated LDL-cholesterol level might benefit from additional LDL-cholesterol lowering therapies, or agents that favourably modify HDL-cholesterol and triglyceride levels
-
Bile acid resins and ezetimibe can be used with or without statins to reduce LDL-cholesterol levels, although the clinical benefit of ezetimibe awaits the results of a randomized clinical trial
-
Patients with homozygous familial hypercholesterolaemia might benefit from lomitapide, a microsomal triglyceride transfer protein inhibitor, and mipomersen, an antisense inhibitor of apolipoprotein B synthesis
-
Niacin and fibrates help correct lipid abnormalities and can reduce cardiovascular risk in patients with a low HDL-cholesterol level, high triglyceride levels, or both
-
Results of clinical trials with experimental cholesteryl ester transfer protein inhibitors are eagerly anticipated, and are expected to provide valuable data on the clinical benefit of pharmacologically raising HDL cholesterol
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Baigent, C. et al. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet 376, 1670–1681 (2010).
Mihaylova, B. et al. The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet 380, 581–590 (2012).
Taylor, F. et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2013, Issue 1. Art. No.: CD004816. http://dx.doi.org/10.1002/14651858.CD004816.pub5.
Kostis, W. J., Cheng, J. Q., Dobrzynski, J. M., Cabrera, J. & Kostis, J. B. Meta-analysis of statin effects in women versus men. J. Am. Coll. Cardiol. 59, 572–582 (2012).
Hovingh, G. K., Davidson, M. H., Kastelein, J. J. & O'Connor, A. M. Diagnosis and treatment of familial hypercholesterolemia. Eur. Heart J. 34, 962–971 (2013).
Fernandez, G., Spatz, E. S., Jablecki, C. & Phillips, P. S. Statin myopathy: a common dilemma not reflected in clinical trials. Cleve. Clin. J. Med. 78, 393–403 (2011).
Hsia, J., MacFadyen, J. G., Monyak, J. & Ridker, P. M. Cardiovascular event reduction and adverse events among subjects attaining low-density lipoprotein cholesterol <50 mg/dl with rosuvastatin. The JUPITER trial (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin). J. Am. Coll. Cardiol. 57, 1666–1675 (2011).
Grundy, S. M. et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. Circulation 110, 227–239 (2004).
Lambert, G., Sjouke, B., Choque, B., Kastelein, J. J. & Hovingh, G. K. The PCSK9 decade. J. Lipid Res. 53, 2515–2524 (2012).
Pinkosky, S. L. et al. AMP-activated protein kinase and ATP-citrate lyase are two distinct molecular targets for ETC-1002, a novel small molecule regulator of lipid and carbohydrate metabolism. J. Lipid Res. 54, 134–151 (2013).
Ballantyne, C. M., et al. Efficacy and safety of a novel dual modulator of adenosine triphosphate-citrate lyase and adenosine monophosphate-activated protein kinase in subjects with hypercholesterolemia: the results of a double-blind, parallel group, multicenter, placebo controlled trial. J. Am. Coll. Cardiol. http://dx.doi.org/10.1016/j.jacc.2013.05.050.
Out, C., Groen, A. K. & Brufau, G. Bile acid sequestrants: more than simple resins. Curr. Opin. Lipidol. 23, 43–55 (2012).
Hou, R. & Goldberg, A. C. Lowering low-density lipoprotein cholesterol: statins, ezetimibe, bile acid sequestrants, and combinations: comparative efficacy and safety. Endocrinol. Metab. Clin. North Am. 38, 79–97 (2009).
Product information. Colestid® (micronized colestipol hydrochloride tablets; Pharmacia & Upjohn Company, Kalamazoo, MI, USA) [online], (2006).
Product information. Welchol® (colesevelam hydrochloride; Daiichi Sankyo, Inc., Parsippany, NJ, USA) [online], (2013).
Ooi, C. P. & Loke, S. C. Colesevelam for type 2 diabetes mellitus. Cochrane Database of Systematic Reviews, Issue 12. Art. No.: CD009361. http://dx.doi.org/10.1002/14651858.CD009361.pub2.
The Lipid Research Clinics Coronary Primary Prevention Trial. Results 1: reduction in incidence of coronary heart disease. JAMA 251, 351–364 (1984).
Hujigen, R. et al. Colesevelam added to combination therapy with a statin and ezetimibe in patients with familial hypercholesterolemia: a 12-week, multicenter, randomized, double-blind, controlled trial. Clin. Ther. 32, 615–625 (2010).
Kawashiri, M. et al. Efficacy and safety of coadministration of rosuvastatin, ezetimibe, and colestimide in heterozygous familial hypercholesterolemia. Am. J. Cardiol. 109, 364–369 (2012).
Wang, L. J. & Song, B. L. Niemann-Pick C1-Like 1 and cholesterol uptake. Biochim. Biophys. Acta 1821, 964–972 (2012).
Dembowski, E. & Davidson, M. H. Statin and ezetimibe combination therapy in cardiovascular disease. Curr. Opin. Endocrinol. Diabetes Obes. 16, 183–188 (2009).
Product information. Zetia® (ezetimibe; MSD International Gmbh, Lucerne, Switzerland) [online], (2013).
Product information. Vytorin® (ezetimibe/simvastatin; MSD International Gmbh, Lucerne, Switzerland) [online], (2013).
Product information. Liptruzet® (ezetimibe/atorvastatin; Merck Sharp & Dohme Corp., Whitehouse Station, NJ, USA) [online], (2013).
Pandor, A. et al. Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials. J. Intern. Med. 265, 568–580 (2009).
Mikhailidis, D. P. et al. Comparative efficacy of the addition of ezetimibe to statin vs statin titration in patients with hypercholesterolemia: systematic review and meta-analysis. Curr. Med. Res. Opin. 27, 1191–1210 (2011).
Kastelein, J. J. et al. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N. Engl. J. Med. 358, 1431–1443 (2008).
Taylor, A. J. et al. Extended-release niacin or ezetimibe and carotid intima–media thickness. N. Engl. J. Med. 361, 2113–2122 (2008).
Fleg, J. L. et al. Effect of statins alone versus statins plus ezetimibe on carotid atherosclerosis in type 2 diabetes: the SANDS (Stop Atherosclerosis in Native Diabetics Study) trial. J. Am. Coll. Cardiol. 52, 2198–2205 (2008).
Meaney, A. et al. The VYtorin on Carotid intima-media thickness and overall arterial rigidity (VYCTOR) study. J. Clin. Pharmacol. 49, 838–847 (2009).
Rossebø, A. B. et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N. Engl. J. Med. 359, 1343–1356 (2008).
Baigent, C. et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet 377, 2181–2192 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2012).
Hussain, M. M. et al. Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44, 22–32 (2003).
Wetterau, J. R. et al. Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 (1992).
Cuchel, M. et al. Inhibition of microsomal triglyceride transfer protein in familial hypercholesterolemia. N. Engl. J. Med. 356, 148–156 (2007).
Samaha, F. F., McKenney, J., Bloedon, L. T., Sasiela, W. J. & Rader, D. J. Inhibition of microsomal triglyceride transfer protein alone or with ezetimibe in patients with moderate hypercholesterolemia. Nat. Clin. Pract. Cardiovasc. Med. 5, 497–505 (2008).
Cuchel, M. et al. Efficacy and safety of a microsomal triglyceride transfer protein inhibitor in patients with homozygous familial hypercholesterolemia: a single-arm, open-label, phase 3 study. Lancet 381, 40–46 (2013).
Product information. Juxtapid® (lomitapide; Aegerion Pharmaceuticals, Inc., Cambridge, MA, USA) [online], (2012).
Toth, P. P. Antisense therapy and emerging applications for the management of dyslipidemia. J. Clin. Lipidol. 5, 441–449 (2011).
Visser, M. E., Witztum, J. L., Stroes, E. S. & Kastelein, J. J. Antisense oligonucleotides for the treatment of dyslipidemia. Eur. Heart J. 33, 1451–1458 (2012).
Product information. Kynamro® (mipomersen sodium; Genzyme Corporation, Cambridge, MA, USA) [online], (2013).
Raal, F. J. et al. Mipomersen, an apolipoprotein B synthesis inhibitor, for lowering of LDL cholesterol concentrations in patients with homozygous familial hypercholesterolemia: a randomized, double-blind, placebo-controlled trial. Lancet 375, 998–1006 (2010).
Stein, E. A. et al. Apolipoprotein synthesis inhibition with mipomersen in heterozygous familial hypercholesterolemia: results of a randomized, double-blind, placebo-controlled trial to assess efficacy and safety as add-on therapy in patients with coronary artery disease. Circulation 126, 2283–2292 (2012).
McGowan, M. P. et al. Randomized, placebo-controlled trial of mipomersen in patients with severe hypercholesterolemia receiving maximally tolerated lipid-lowering therapy. PLoS ONE 7, e49006 (2012).
FDA Briefing Document. NDA 203568. Mipomersen sodium injection 200 mg/ml. Endocrinologic and Metabolic Drugs Advisory Committee meeting, October 18 2012 [online], (2012).
Wilson, P. W., Abbott, R. D. & Castelli, W. P. High density lipoprotein cholesterol and mortality. The Framingham Heart Study. Arteriosclerosis 8, 737–741 (1988).
Canner, P. L. et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J. Am. Coll. Cardiol. 8, 1245–1255 (1986).
Frick, M. H. et al. Helsinki Heart Study: primary prevention trial with gemfibrozil in middle-aged men with dyslipidemia. Safety of treatment, changes in risk factors, and incidence of coronary heart disease. N. Engl. J. Med. 317, 1237–1245 (1987).
Rubins, H. B. et al. Gemfibrozil for the secondary prevention of coronary heart disease in men with low levels of high-density lipoprotein cholesterol. Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial Study Group. N. Engl. J. Med. 341, 410–418 (1999).
Boden, W. E. et al. Niacin in patients with low HDL-cholesterol levels receiving intensive statin therapy. N. Engl. J. Med. 365, 2255–2267 (2011).
Armitage, J. M. HPS2-THRIVE: Randomized comparison of extended-release (ER) niacin/laropiprant 2 g daily versus placebo in 25,673 patients at high risk of occlusive vascular events. Presented at ACC Scientific Sessions 2013.
Keech, A. et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomized controlled trial. Lancet 366, 1849–1861 (2005).
Ginsberg, H. N. et al. Effects of combination lipid therapy in type 2 diabetes mellitus. N. Engl. J. Med. 362, 1563–1574 (2010).
Barter, P. J. et al. Effects of torcetrapib in patients at high risk for coronary events. N. Engl. J. Med. 357, 2109–2122 (2007).
Schwartz, G. G. et al. Effects of dalcetrapib in patients with a recent acute coronary syndrome. N. Engl. J. Med. 367, 2089–2099 (2012).
Voight, B. F. et al. Plasma HDL cholesterol and risk of myocardial infarction: a Mendelian randomisation study. Lancet 380, 572–580 (2012).
Barter, P. et al. HDL cholesterol, very low levels of LDL cholesterol, and cardiovascular events. N. Engl. J. Med. 357, 1301–1310 (2007).
Ridker, P. M. et al. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: an analysis from the JUPITER trial. Lancet 376, 333–339 (2010).
Otocka-Kmiecik, A. et al. Dysfunctional HDL: a novel important diagnostic and therapeutic target in cardiovascular disease? Prog. Lipid Res. 51, 314–324 (2012).
Khera, A. V. et al. Cholesterol efflux capacity, high-density lipoprotein function, and atherosclerosis. N. Engl. J. Med. 364, 127–135 (2011).
Chapman, M. J. et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Eur. Heart J. 32, 1345–1361 (2011).
Miller, M. et al. Triglycerides and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 123, 2292–2333 (2011).
Digby, J. E., Ruparelia, N. & Choudhury, R. P. Niacin in cardiovascular disease: recent preclinical and clinical developments. Arterioscler. Thromb. Vasc. Biol. 32, 582–588 (2012).
Lauring, B. et al. Niacin lipid efficacy is independent of both the niacin receptor GPR109A and free fatty acid suppression. Sci. Transl. Med. 4, 148ra115 (2012).
MacKay, D., Hathcock, J. & Guarneri, E. Niacin: chemical forms, bioavailability, and health effects. Nutr. Rev. 70, 357–366 (2012).
Product information. Niaspan® (niacin extended-release; Abbvie Respiratory LLC, North Chicago, IL, USA) [online], (2013).
Brinton, E. A. et al. Niacin extended-release therapy in phase III clinical trials is associated with relatively low rates of drug discontinuation due to flushing and treatment-related adverse events: a pooled analysis. Am. J. Cardiovasc. Drugs 11, 179–187 (2011).
Kamanna, V. S., Ganji, S. H. & Kashyap, M. L. The mechanism and mitigation of niacin-induced flushing. Int. J. Clin. Pract. 63, 1369–1377 (2009).
Maccubbin, D. L. et al. Effectiveness and safety of laropiprant on niacin-induced flushing. Am. J. Cardiol. 110, 817–822 (2012).
HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25,673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur. Heart J. 34, 1279–1291 (2013).
Brown, B. G., Canner, P. L., McGovern, M., Guyton, J. R. & Carlson, L. A. In Clinical Lipidology: A Companion to Braunwald's Heart Disease (ed. Ballantyne, C. M.) 298–314 (Elsevier, 2008).
Bruckert, E., Labreuche, J. & Amarenco, P. Meta-analysis of the effect of nicotinic acid alone or in combination on cardiovascular events and atherosclerosis. Atherosclerosis 210, 353–361 (2010).
Shah, A., Rader, D. J. & Millar, J. S. The effect of PPAR-alpha agonism on apolipoprotein metabolism in humans. Atherosclerosis 210, 35–40 (2010).
Product information. TriCor® (fenofibrate; Abbott Laboratories, Abbott Park, IL, USA) [online], (2013).
Product information. Lopid® (gemfibrozil; Warner-Lambert Company, New York, NY, USA) [online], (2010).
Product information. Trilipix® (fenofibric acid; Fournier Industrie Et Sante' Corporation, Dijon, France) [online], (2012).
Effect of fenofibrate on progression of coronary-artery disease in type 2 diabetes: the Diabetes Atherosclerosis Intervention Study, a randomized study. Lancet 357, 905–910 (2001).
Scott, R. et al. Effects of fenofibrate treatment on cardiovascular disease risk in 9,795 individuals with type 2 diabetes and various components of the metabolic syndrome: the Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study. Diabetes Care 32, 493–498 (2009).
The BIP Study Group. Secondary prevention by raising HDL cholesterol and reducing triglycerides in patients with coronary artery disease: the Bezafibrate Infarction Prevention (BIP) study. Circulation 102, 21–27 (2000).
Manninen, V. Joint effects of serum triglyceride and LDL cholesterol and HDL cholesterol concentrations on coronary heart disease risk in the Helsinki Heart Study. Implications for treatment. Circulation 85, 37–45 (1992).
Jun, M. et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 375, 1875–1884 (2010).
Barter, P. J. et al. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 23, 160–167 (2003).
Inazu, A. et al. Increased high-density lipoprotein levels caused by a common cholesteryl-ester transfer protein gene mutation. N. Engl. J. Med. 323, 1234–1238 (1990).
Inazu, A. et al. Genetic cholesteryl ester transfer protein deficiency caused by two prevalent mutations as a major determinant of increased levels of high density lipoprotein cholesterol. J. Clin. Invest. 94, 1872–1882 (1994).
Thompson, A. et al. Association of cholesteryl ester transfer protein genotypes with CETP mass and activity, lipid levels, and coronary risk. JAMA 299, 2777–2788 (2008).
Ridker, P. M. et al. Polymorphism in the CETP gene region, HDL cholesterol, and risk of future myocardial infarction: Genomewide analysis among 18,245 initially healthy women from the Women's Genome Health Study. Circ. Cardiovasc. Genet. 2, 26–33 (2009).
Johannsen, T. H., Frikke-Schmidt, R., Schou, J., Nordestgaard, B. G. & Tybjærg-Hansen, A. Genetic inhibition of CETP, ischemic vascular disease and mortality, and possible adverse effects. J. Am. Coll. Cardiol. 60, 2041–2048 (2012).
Zhong, S. et al. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J. Clin. Invest. 97, 2917–2923 (1996).
Agerholm-Larsen, B., Nordestgaard, B. G., Steffensen, R., Jensen, G. & Tybjaerg-Hansen, A. Elevated HDL cholesterol is a risk factor for ischemic heart disease in white women when caused by a common mutation in the cholesteryl ester transfer protein gene. Circulation 101, 1907–1912 (2000).
Vasan, R. S. et al. Association of circulating cholesteryl ester transfer protein activity with incidence of cardiovascular disease in the community. Circulation 120, 2414–2420 (2009).
Fayad, Z. A. et al. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised trial. Lancet 378, 1547–1559 (2011).
Lüscher, T. F. et al. Vascular effects and safety of dalcetrapib in patients with or at risk of coronary heart disease: the dal-VESSEL randomized clinical trial. Eur. Heart J. 33, 857–865 (2012).
Ranalletta, M. et al. Biochemical characterization of cholesteryl ester transfer protein inhibitors. J. Lipid Res. 51, 2739–2752 (2010).
Niesor, E. J. et al. Modulating cholesteryl ester transfer protein activity maintains efficient pre-ß-HDL formation and increases reverse cholesterol transport. J. Lipid Res. 51, 3443–3454 (2010).
Niesor, E. J. Different effects of compounds decreasing cholesteryl ester transfer protein activity on lipoprotein metabolism. Curr. Opin. Lipidol. 22, 288–295 (2011).
Gutstein, D. E. et al. Anacetrapib, a novel CETP inhibitor: pursuing a new approach to cardiovascular risk reduction. Clin. Pharmacol. Ther. 91, 109–122 (2012).
Yvan-Charvet, L. et al. Cholesterol efflux potential and anti-inflammatory properties of high-density lipoprotein after treatment with niacin or anacetrapib. Arterioscler. Thromb. Vasc. Biol. 30, 1430–1438 (2010).
Castro-Perez, J. et al. Anacetrapib promotes reverse cholesterol transport and bulk cholesterol excretion in Syrian golden hamsters. J. Lipid Res. 52, 1965–1973 (2011).
Cannon, C. P. et al. Safety of anacetrapib in patient with or at high risk for coronary heart disease. N. Engl. J. Med. 363, 2406–2415 (2010).
Davidson, M. et al. Measurement of LDL-C after treatment with the CETP inhibitor anacetrapib. J. Lipid Res. 54, 467–472 (2013).
Gotto, A. M. Jr et al. Effects on lipids and safety following cessation of treatment with cholesteryl ester transfer protein inhibitor anacetrapib in patients with or at high risk for coronary heart disease [abstract 15035]. Circulation 124, A15035 (2011).
Cao, G. et al. Evacetrapib is a novel, potent and selective inhibitor of cholesteryl ester transfer protein that elevates HDL cholesterol without inducing aldosterone or increasing blood pressure. J. Lipid Res. 52, 2169–2176 (2011).
Nicholls, S. J. et al. Effects of the CETP inhibitor evacetrapib administered as monotherapy or in combination with statins on HDL and LDL cholesterol: a randomized controlled trial. JAMA 306, 2099–2109 (2011).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
US National Library of Medicine. ClinicalTrials.gov [online], (2013).
Shaw, J. A. et al. Infusion of reconstituted high-density lipoprotein leads to acute changes in human atherosclerotic plaque. Circ. Res. 103, 1084–1091 (2008).
Waksman, R. et al. A first-in-man, randomized, placebo-controlled study to evaluate the safety and feasibility of autologous delipidated high-density lipoprotein plasma infusions in patients with acute coronary syndrome. J. Am. Coll. Cardiol. 55, 2727–2735 (2010).
Nicholls, S. J. et al. Relationship between atheroma regression and change in lumen size after infusion of apolipoprotein A-I Milano. J. Am. Coll. Cardiol. 47, 992–997 (2006).
Hovingh, G. K., Bochem, A. E. & Kastelein, J. J. Apolipoprotein A-I mimetic peptides. Curr. Opin. Lipidol. 21, 481–486 (2010).
Davidson, M. H. Apolipoprotein A-I therapy promise, challenges, and disappointment. J. Am. Coll. Cardiol. 57, 1120–1121 (2011).
Bailey, D. et al. RVX-208: a small molecule that increases apolipoprotein A-I and high-density lipoprotein cholesterol in vitro and in vivo. J. Am. Coll. Cardiol. 55, 2580–2589 (2010).
Nicholls, S. J. et al. Efficacy and safety of a novel oral inducer of apolipoprotein A-I synthesis in statin-treated patients with stable coronary artery disease a randomized controlled trial. J. Am. Coll. Cardiol. 57, 1111–1119 (2011).
Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 285, 2486–2497 (2001).
Author information
Authors and Affiliations
Contributions
Both authors contributed to researching data for article, discussion of content, writing the article, and reviewing/editing the article before submission.
Corresponding author
Ethics declarations
Competing interests
A. M. Gotto is a stockholder/ Director of Aegerion and Arisaph Pharmaceuticals; he has acted as a consultant for AstraZeneca, Janssen, Kowa, Merck, and Roche; and is on the advisory board of DuPont and Vatera Capital. J. E. Moon declares no competing interests.
Rights and permissions
About this article
Cite this article
Gotto, A., Moon, J. Pharmacotherapies for lipid modification: beyond the statins. Nat Rev Cardiol 10, 560–570 (2013). https://doi.org/10.1038/nrcardio.2013.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrcardio.2013.117
This article is cited by
-
Causes, clinical findings and therapeutic options in chylomicronemia syndrome, a special form of hypertriglyceridemia
Lipids in Health and Disease (2022)
-
Changes in triglyceride, HDL-C, and non-HDL-C levels in patients with acute coronary syndrome
Wiener klinische Wochenschrift (2016)
-
Chylomicronaemia—current diagnosis and future therapies
Nature Reviews Endocrinology (2015)
-
A Current Approach to Statin Intolerance
Clinical Pharmacology & Therapeutics (2014)
-
Therapie von Fettstoffwechselstörungen
Herz (2014)