Key Points
-
Preventative vaccination against infectious organisms has had a dramatic effect on public health. Therapeutic vaccination against cancer is more challenging but, armed with new immunological insight and genetic technology, aims similarly to harness the power of the immune system, in this case to destroy or suppress tumour cells.
-
Passively transferred antibodies and T cells are clearly able to attack human cancer cells in vivo and are included in treatment protocols for some cancers. Active vaccination would generate these effector pathways, together with immunological memory that is able to continuously detect and remove any emergent cancer cells.
-
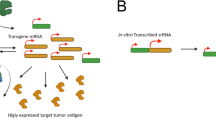
Tumour antigens are being rapidly revealed, and can be expressed on cell surfaces or, more commonly, as peptides in association with the major histocompatibility complex class I (or II) molecules. DNA vaccines can be designed to activate antibody and/or T-cell responses, providing focused immune attack on selected antigens.
-
DNA vaccines offer a precise but flexible strategy for delivering antigens to the immune system, and additional sequences encoding molecules to manipulate outcome can be included. The problem of translating success in preclinical models to patients seems to be overcome by using electroporation, which dramatically improves performance and is now in clinical trials for prostate cancer.
-
The key to bypassing immune tolerance and activating high levels of anti-tumour antibody or cytotoxic T cells lies in inducing CD4+ T-cell help. Sequences derived from microbial antigens can be incorporated into anti-tumour DNA vaccines, a strategy which mobilizes help for anti-tumour responses from the large non-tolerized anti-microbial repertoire.
-
Clinical trials are the real test, but the current question is largely of efficacy rather than toxicity. New thinking is informing pilot trial design within a highly regulated environment, and immunological assays that can be predictors of clinical outcome are developing rapidly.
Abstract
DNA vaccination has suddenly become a favoured strategy for inducing immunity. The molecular precision offered by gene-based vaccines, together with the facility to include additional genes to direct and amplify immunity, has always been attractive. However, the apparent failure to translate operational success in preclinical models to the clinic, for reasons that are now rather obvious, reduced initial enthusiasm. Recently, novel delivery systems, especially electroporation, have overcome this translational block. Here, we assess the development, current performance and potential of DNA vaccines for the treatment of cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Stanley, M. Prophylactic HPV vaccines. J. Clin. Pathol. 60, 961–965 (2007).
Maloney, D. G. et al. Phase I clinical trial using escalating single-dose infusion of chimeric anti-CD20 monoclonal antibody (IDEC-C2B8) in patients with recurrent B-cell lymphoma. Blood 84, 2457–2466 (1994).
Valone, F. H. et al. Clinical trials of bispecific antibody MDX-210 in women with advanced breast or ovarian cancer that overexpresses HER-2/neu. J. Hematother 4, 471–475 (1995).
Goldman, J. M. et al. Bone marrow transplantation for chronic myelogenous leukemia in chronic phase. Increased risk for relapse associated with T-cell depletion. Ann. Intern. Med. 108, 806–814 (1988).
Fontaine, P. et al. Adoptive transfer of minor histocompatibility antigen-specific T lymphocytes eradicates leukemia cells without causing graft-versus-host disease. Nature Med. 7, 789–794 (2001).
George, A. J. & Stevenson, F. K. Prospects for the treatment of B cell tumors using idiotypic vaccination. Int. Rev. Immunol. 4, 271–310 (1989).
Van Der Bruggen, P. et al. Tumor-specific shared antigenic peptides recognized by human T cells. Immunol. Rev. 188, 51–64 (2002).
Stevenson, F. K., Rice, J. & Zhu, D. Tumor vaccines. Adv. Immunol. 82, 49–103 (2004).
Galon, J. et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313, 1960–1964 (2006).
Darnell, R. B. & Posner, J. B. Observing the invisible: successful tumor immunity in humans. Nat. Immunol. 4, 201 (2003).
Curiel, T. J. Tregs and rethinking cancer immunotherapy. J. Clin. Invest. 117, 1167–1174 (2007).
Lutsiak, M. E. et al. Inhibition of CD4+25+ T regulatory cell function implicated in enhanced immune response by low-dose cyclophosphamide. Blood 105, 2862–2868 (2005).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Munn, D. H. & Mellor, A. L. Indoleamine 2,3-dioxygenase and tumor-induced tolerance. J. Clin. Invest. 117, 1147–1154 (2007).
Emens, L. A., Reilly, R. T. & Jaffee, E. M. Breast cancer vaccines: maximizing cancer treatment by tapping into host immunity. Endocr. Relat. Cancer 12, 1–17 (2005).
Dell'Agnola, C. & Biragyn, A. Clinical utilization of chemokines to combat cancer: the double-edged sword. Expert Rev. Vaccines 6, 267–283 (2007).
Coscia, M. & Biragyn, A. Cancer immunotherapy with chemoattractant peptides. Semin. Cancer Biol. 14, 209–218 (2004).
Calarota, S. A. & Weiner, D. B. Enhancement of human immunodeficiency virus type 1-DNA vaccine potency through incorporation of T-helper 1 molecular adjuvants. Immunol. Rev. 199, 84–99 (2004).
Zhu, D. & Stevenson, F. K. DNA gene fusion vaccines against cancer. Curr. Opin. Mol. Ther. 4, 41–48 (2002).
Kim, T. W. et al. Vaccination with a DNA vaccine encoding herpes simplex virus type 1 VP22 linked to antigen generates long-term antigen-specific CD8-positive memory T cells and protective immunity. Hum. Gene Ther. 15, 167–177 (2004).
Perkins, S. D. et al. VP22 enhances antibody responses from DNA vaccines but not by intercellular spread. Vaccine 23, 1931–1940 (2005).
Gurunathan, S., Klinman, D. M. & Seder, R. A. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18, 927–974 (2000).
Hoebe, K. et al. Genetic analysis of innate immunity. Adv. Immunol. 91, 175–226 (2006).
Walter, P. & Johnson, A. E. Signal sequence recognition and protein targeting to the endoplasmic reticulum membrane. Annu. Rev. Cell Biol. 10, 87–119 (1994).
Boyle, J. S., Koniaras, C. & Lew, A. M. Influence of cellular location of expressed antigen on the efficacy of DNA vaccination: cytotoxic T lymphocyte and antibody responses are suboptimal when antigen is cytoplasmic after intramuscular DNA immunization. Int. Immunol. 9, 1897–1906 (1997).
Rice, J. et al. Manipulation of pathogen-derived genes to influence antigen presentation via DNA vaccines. Vaccine 17, 3030–3038 (1999).
Bacik, I. et al. Introduction of a glycosylation site into a secreted protein provides evidence for an alternative antigen processing pathway: transport of precursors of major histocompatibility complex class I-restricted peptides from the endoplasmic reticulum to the cytosol. J. Exp. Med. 186, 479–487 (1997).
Stevenson, F. K. et al. DNA vaccines to attack cancer. Proc. Natl Acad. Sci. USA 101 (Suppl. 2), 14646–14652 (2004).
Leifert, J. A., Rodriguez-Carreno, M. P., Rodriguez, F. & Whitton, J. L. Targeting plasmid-encoded proteins to the antigen presentation pathways. Immunol. Rev. 199, 40–53 (2004).
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 (1998).
Stoitzner, P. et al. Langerhans cells cross-present antigen derived from skin. Proc. Natl Acad. Sci. USA 103, 7783–7788 (2006).
Shirota, H., Petrenko, L., Hong, C. & Klinman, D. M. Potential of transfected muscle cells to contribute to DNA vaccine immunogenicity. J. Immunol. 179, 329–336 (2007).
Heath, W. R. et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol. Rev. 199, 9–26 (2004).
Radcliffe, J. N. et al. Prime-boost with alternating DNA vaccines designed to engage different antigen presentation pathways generates high frequencies of peptide-specific CD8+ T cells. J. Immunol. 177, 6626–6633 (2006).
Rice, J., Elliott, T., Buchan, S. & Stevenson, F. K. DNA fusion vaccine designed to induce cytotoxic T cell responses against defined peptide motifs: implications for cancer vaccines. J. Immunol. 167, 1558–1565 (2001).
Guermonprez, P. et al. ER-phagosome fusion defines an MHC class I cross-presentation compartment in dendritic cells. Nature 425, 397–402 (2003).
Villadangos, J. A., Heath, W. R. & Carbone, F. R. Outside looking in: the inner workings of the cross-presentation pathway within dendritic cells. Trends Immunol. 28, 45–47 (2007).
Delamarre, L. et al. Differential lysosomal proteolysis in antigen-presenting cells determines antigen fate. Science 307, 1630–1634 (2005).
Bourgeois, C. et al. CD8 lethargy in the absence of CD4 help. Eur. J. Immunol. 32, 2199–2207 (2002).
Shedlock, D. J. & Shen, H. Requirement for CD4 T cell help in generating functional CD8 T-cell memory. Science 300, 337–339 (2003).
Sun, J. C. & Bevan, M. J. Defective CD8 T-cell memory following acute infection without CD4 T-cell help. Science 300, 339–342 (2003).
Janssen, E. M. et al. CD4+ T cells are required for secondary expansion and memory in CD8+ T lymphocytes. Nature 421, 852–856 (2003). This paper establishes that, although a primary CD8+ T-cell response may be independent of CD4+ T-cell help, the essential secondary CTL expansion occurring on re-encounter with antigen is wholly dependent on provision of T-cell help at the time of priming. The requirement for CD4+ T-cell dependent programming of CD8+ T-cell responses must be met by vaccines aimed against cancer.
Melief, C. J. et al. Effective therapeutic anticancer vaccines based on precision guiding of cytolytic T lymphocytes. Immunol. Rev. 188, 177–182 (2002).
Lu, Z. et al. CD40-independent pathways of T cell help for priming of CD8+ cytotoxic T lymphocytes. J. Exp. Med. 191, 541–550 (2000).
Behrens, G. M. et al. Helper requirements for generation of effector CTL to islet β cell antigens. J. Immunol. 172, 5420–5426 (2004).
Hernandez, J., Aung, S., Marquardt, K. & Sherman, L. A. Uncoupling of proliferative potential and gain of effector function by CD8+ T cells responding to self-antigens. J. Exp. Med. 196, 323–333 (2002).
Tan, R. et al. Rapid death of adoptively transferred T cells in acquired immunodeficiency syndrome. Blood 93, 1506–1510 (1999).
Hamilton, S. E., Wolkers, M. C., Schoenberger, S. P. & Jameson, S. C. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 7, 475–481 (2006).
Williams, M. A., Tyznik, A. J. & Bevan, M. J. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature 441, 890–893 (2006).
Janssen, E. M. et al. CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434, 88–93 (2005).
Prlic, M., Williams, M. A. & Bevan, M. J. Requirements for CD8 T-cell priming, memory generation and maintenance. Curr. Opin. Immunol. 19, 315–319 (2007).
Wolkers, M. C. et al. Optimizing the efficacy of epitope-directed DNA vaccination. J. Immunol. 168, 4998–5004 (2002).
Savelyeva, N. et al. Plant viral genes in DNA idiotypic vaccines activate linked CD4+ T-cell mediated immunity against B-cell malignancies. Nature Biotechnol. 19, 760–764 (2001).
Hung., C. F. et al. Cancer immunotherapy using a DNA vaccine encoding the translocation domain of a bacterial toxin linked to a tumor antigen. Cancer Res. 61, 3698–3703. (2001).
Stevenson, F. K. et al. DNA fusion gene vaccines against cancer: from the laboratory to the clinic. Immunol. Rev. 199, 156–180 (2004).
Bergman, P. J. et al. Development of a xenogeneic DNA vaccine program for canine malignant melanoma at the Animal Medical Center. Vaccine 24, 4582–4585 (2006).
Guevara-Patino, J. A., Turk, M. J., Wolchok, J. D. & Houghton, A. N. Immunity to cancer through immune recognition of altered self: studies with melanoma. Adv. Cancer Res. 90, 157–177 (2003).
Bergman, P. J. et al. Long-term survival of dogs with advanced malignant melanoma after DNA vaccination with xenogeneic human tyrosinase: a phase I trial. Clin. Cancer Res. 9, 1284–1290 (2003).
Nakajima, C. et al. A role of interferon-γ (IFN- γ) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN- γ -deficient mice. Cancer Res. 61, 3399–3405 (2001).
Wilcox, R. A. et al. Impaired infiltration of tumor-specific cytolytic T cells in the absence of interferon-γ despite their normal maturation in lymphoid organs during CD137 monoclonal antibody therapy. Cancer Res. 62, 4413–4418 (2002).
Carter, P. J. Potent antibody therapeutics by design. Nature Rev. Immunol. 6, 343–357 (2006).
Hamblin, T. J. et al. Preliminary experience in treating lymphocytic leukaemia with antibody to immunoglobulin idiotypes on the cell surfaces. Br. J. Cancer 42, 495–502 (1980).
Lowder, J. N. et al. Studies on B lymphoid tumors treated with monoclonal anti-idiotype antibodies: correlation with clinical responses. Blood 69, 199–210 (1987).
Timmerman, J. M. et al. Immunogenicity of a plasmid DNA vaccine encoding chimeric idiotype in patients with B-cell lymphoma. Cancer Res. 62, 5845–5852 (2002).
Stevenson, F. K. et al. A genetic approach to idiotypic vaccination for B cell lymphoma. Ann. NY Acad. Sci. 772, 212–226 (1995).
Spellerberg, M. B. et al. DNA vaccines against lymphoma: promotion of anti-idiotypic antibody responses induced by single chain Fv genes by fusion to tetanus toxin fragment, C. J. Immunol. 159, 1885–1892 (1997).
King, C. A. et al. DNA vaccines with single-chain Fv fused to fragment C of tetanus toxin induce protective immunity against lymphoma and myeloma. Nature Med. 4, 1281–1286 (1998). The concept of using a microbial sequence fused to an antigen sequence to amplify antibody responses also operates through DNA delivery. Fusion converted a poorly immunogenic idiotypic antigen into a powerful immunogen able to provide specific protection against lymphoma.
Savelyeva, N., King, C. A., Vitetta, E. S. & Stevenson, F. K. Inhibition of a vaccine-induced anti-tumor B cell response by soluble protein antigen in the absence of continuing T cell help. Proc. Natl Acad. Sci. USA 102, 10987–10992 (2005).
Padua, R. A. et al. PML-RARA-targeted DNA vaccine induces protective immunity in a mouse model of leukemia. Nature Med. 9, 1413–1417 (2003).
Smith, C. M. et al. Cognate CD4+ T cell licensing of dendritic cells in CD8+ T cell immunity. Nat. Immunol. 5, 1143–1148 (2004).
Gerloni, M., Castiglioni, P. & Zanetti, M. The cooperation between two CD4 T cells induces tumor protective immunity in MUC.1 transgenic mice. J. Immunol. 175, 6551–6559 (2005). Weak CD4+ T-cell responses can themselves be helped by parallel CD4+ T-cell responses to foreign epitopes. This phenomenon of T H –T H cooperation might be particularly important in overcoming regulation and again informs vaccine design.
Mueller, S. N. et al. CD4+ T cells can protect APC from CTL-mediated elimination. J. Immunol. 176, 7379–7384 (2006).
Yewdell, J. W. & Bennink, J. R. Immunodominance in major histocompatibility complex class I-restricted T lymphocyte responses. Ann. Rev. Immunol. 17, 51–88 (1999).
Chen, W. & McCluskey, J. Immunodominance and immunodomination: critical factors in developing effective CD8+ T-cell-based cancer vaccines. Adv. Cancer Res. 95, 203–247 (2006). Immunodominance is a natural focusing of CTL activity against a few target epitopes. Responses against strong epitopes can suppress those against weak epitopes and could allow tumour cells to escape.
Le, T. T. et al. Cytotoxic T cell polyepitope vaccines delivered by ISCOMs. Vaccine 19, 4669–4675 (2001).
Smith, S. G. et al. Human dendritic cells genetically engineered to express a melanoma polyepitope DNA vaccine induce multiple cytotoxic T-cell responses. Clin. Cancer Res. 7, 4253–4261 (2001).
Rice, J., Buchan, S. & Stevenson, F. K. Critical components of a DNA fusion vaccine able to induce protective cytotoxic T cells against a single epitope of a tumor antigen. J. Immunol. 169, 3908–3913 (2002). A DNA vaccine design using a minimized microbial sequence to provide non-immunodominating T-cell help, together with a single tumour-derived epitope to generate focused CD8+ T-cell responses, appears effective. It is now in clinical trials.
Perez-Diez, A. et al. Intensity of the vaccine-elicited immune response determines tumor clearance. J. Immunol. 168, 338–347 (2002).
Snyder, H. L. et al. Promiscuous liberation of MHC-class I-binding peptides from the C termini of membrane and soluble proteins in the secretory pathway. Eur. J. Immunol. 28, 1339–1346 (1998).
Rice, J. et al. DNA fusion vaccines induce targeted epitope-specific CTLs against minor histocompatibility antigens from a normal or tolerized repertoire. J. Immunol. 173, 4492–4499 (2004).
Rice, J. et al. DNA fusion vaccines induce epitope-specific cytotoxic CD8+ T cells against human leukemia-associated minor histocompatibility antigens. Cancer Res. 66, 5436–5442 (2006).
van Bergen, J. et al. Efficient loading of HLA-DR with a T helper epitope by genetic exchange of CLIP. Proc. Natl Acad. Sci. USA 94, 7499–7502. (1997).
Firat, H. et al. H-2 class I knockout, HLA-A2.1-transgenic mice: a versatile animal model for preclinical evaluation of antitumor immunotherapeutic strategies. Eur. J. Immunol. 29, 3112–3121 (1999).
Fuller, D. H., Loudon, P. & Schmaljohn, C. Preclinical and clinical progress of particle-mediated DNA vaccines for infectious diseases. Methods 40, 86–97 (2006).
Dupuis, M. et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J. Immunol. 165, 2850–2858. (2000).
McConkey, S. J. et al. Enhanced T-cell immunogenicity of plasmid DNA vaccines boosted by recombinant modified vaccinia virus Ankara in humans. Nature Med. 9, 729–735 (2003). DNA vaccines in a prime–boost combination with a recombinant vaccinia virus were tested in normal human subjects, providing important early data on protection against infection with malaria.
Schneider, J. et al. Enhanced immunogenicity for CD8+ T-cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nature Med. 4, 397–402 (1998).
Moore, A. C. & Hill, A. V. Progress in DNA-based heterologous prime–boost immunization strategies for malaria. Immunol. Rev. 199, 126–143 (2004).
Ramshaw, I. A. & Ramsay, A. J. The prime/boost strategy: exciting prospects for improved vaccination. Immunol. Today 21, 163–165. (2000).
Zavala, F. et al. A striking property of recombinant poxviruses: efficient inducers of in vivo expansion of primed CD8+ T cells. Virology 280, 155–159 (2001).
Kundig, T. M., Kalberer, C. P., Hengartner, H. & Zinkernagel, R. M. Vaccination with two different vaccinia recombinant viruses: long-term inhibition of secondary vaccination. Vaccine 11, 1154–1158 (1993).
Cooney, E. L. et al. Safety of and immunological response to a recombinant vaccinia virus vaccine expressing HIV envelope glycoprotein. Lancet 337, 567–572 (1991).
Rooney, J. F. et al. Immunization with a vaccinia virus recombinant expressing herpes simplex virus type 1 glycoprotein D: long-term protection and effect of revaccination. J. Virol. 62, 1530–1534 (1988).
Smith, C. L. et al. Immunodominance of poxviral-specific CTL in a human trial of recombinant-modified vaccinia Ankara. J. Immunol. 175, 8431–8437 (2005).
Bins, A. D. et al. A rapid and potent DNA vaccination strategy defined by in vivo monitoring of antigen expression. Nature Med. 11, 899–904 (2005).
Greenland, J. R. & Letvin, N. L. Chemical adjuvants for plasmid DNA vaccines. Vaccine 25, 3731–3741 (2007).
Aihara, H. & Miyazaki, J. Gene transfer into muscle by electroporation in vivo. Nature Biotechnol. 16, 867–870 (1998).
Mathiesen, I. Electropermeabilization of skeletal muscle enhances gene transfer in vivo. Gene Ther. 6, 508–514 (1999).
Mir, L. M. et al. High-efficiency gene transfer into skeletal muscle mediated by electric pulses. Proc. Natl Acad. Sci. USA 96, 4262–4267 (1999).
Ahlen, G. et al. In vivo electroporation enhances the immunogenicity of hepatitis C virus nonstructural 3/4A DNA by increased local DNA uptake, protein expression, inflammation, and infiltration of CD3+ T cells. J. Immunol. 179, 4741–4753 (2007). The perceived difficulty in translating the effectiveness of DNA vaccination from pre-clinical rodents to large animals, including human subjects, appears to have been overcome by the use of electroporation. The mechanisms of amplification of performance include increased antigen expression and development of inflammation at the injection site.
Widera, G. et al. Increased DNA vaccine delivery and immunogenicity by electroporation in vivo. J. Immunol. 164, 4635–4640. (2000).
Babiuk, S. et al. Electroporation improves the efficacy of DNA vaccines in large animals. Vaccine 20, 3399–3408 (2002).
Tollefsen, S. et al. Improved cellular and humoral immune responses against Mycobacterium tuberculosis antigens after intramuscular DNA immunisation combined with muscle electroporation. Vaccine 20, 3370–3378 (2002).
Buchan, Sarah et al. Electroporation as a “prime/boost” strategy for naked DNA vaccination against a tumor antigen. J. Immunol. 174, 6292–6298 (2005).
Smith, H. A. & Klinman, D. M. The regulation of DNA vaccines. Curr. Opin. Biotechnol. 12, 299–303. (2001).
Wang, Z. et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 11, 711–721 (2004).
Schalk, J. A. et al. Preclinical and clinical safety studies on DNA vaccines. Hum. Vaccin 2, 45–53 (2006).
Rosenberg, S. A., Yang, J. C. & Restifo, N. P. Cancer immunotherapy: moving beyond current vaccines. Nature Med. 10, 909–915 (2004).
Lowe, D. B., Shearer, M. H., Jumper, C. A. & Kennedy, R. C. Towards progress on DNA vaccines for cancer. Cell. Mol. Life Sci. 64, 2391–2403 (2007).
Todorova, K. et al. Humoral immune response in prostate cancer patients after immunization with gene-based vaccines that encode for a protein that is proteasomally degraded. Cancer Immun. 5, 1 (2005).
Klencke, B. et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clin. Cancer Res. 8, 1028–1037 (2002).
Wolchok, J. D. et al. Safety and immunogenicity of tyrosinase DNA vaccines in patients with melanoma. Mol. Ther. 15, 2044–2050 (2007).
Coleman, S. et al. Recovery of CD8+ T-cell function during systemic chemotherapy in advanced ovarian cancer. Cancer Res. 65, 7000–7006 (2005).
Arlen, P. M. et al. A randomized phase II study of concurrent docetaxel plus vaccine versus vaccine alone in metastatic androgen-independent prostate cancer. Clin. Cancer Res. 12, 1260–1269 (2006).
Ghiringhelli, F. et al. Metronomic cyclophosphamide regimen selectively depletes CD4+CD25+ regulatory T cells and restores T and NK effector functions in end-stage cancer patients. Cancer Immunol. Immunother. 56, 641–648 (2007).
Suzuki, E. et al. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005).
Suzuki, E. et al. Gemcitabine has significant immunomodulatory activity in murine tumor models independent of its cytotoxic effects. Cancer Biol. Ther. 6, (2007).
Kaufman, H. L. & Divgi, C. R. Optimizing prostate cancer treatment by combining local radiation therapy with systemic vaccination. Clin. Cancer Res. 11, 6757–6762 (2005).
Rapoport, A. P. et al. Restoration of immunity in lymphopenic individuals with cancer by vaccination and adoptive T-cell transfer. Nature Med. 11, 1230–1237 (2005).
Stevenson, F. K., Di Genova, G., Ottensmeier, C. H. & Savelyeva, N. in Cancer Immunotherapy: Immune Suppression and Tumor Growth (eds Prendergast, G. C. & Jaffee, E. M.) 183–204 (Academic, London, 2007).
Di Genova, G., Roddick, J., McNicholl, F. & Stevenson, F. K. Vaccination of human subjects expands both specific and bystander memory T cells but antibody production remains vaccine specific. Blood 107, 2806–2813 (2006). A surprising finding emerging from simple studies of CD4+ T-cell responses in normal human subjects to conventional vaccines was that vaccination co-stimulates memory CD4+ T cells against non-vaccine antigens. This 'bystander' stimulation is relevant for the maintenance of T-cell memory and for evaluating vaccine-induced responses.
Mayer, S. et al. Analysis of the immune response against tetanus toxoid: enumeration of specific T helper cells by the ELISPOT assay. Immunobiology 205, 282–289 (2002).
Rahman, F. et al. Cellular and humoral immune responses induced by intradermal or intramuscular vaccination with the major hepatitis B surface antigen. Hepatology 31, 521–527 (2000).
Bocher, W. O. et al. Kinetics of hepatitis B surface antigen-specific immune responses in acute and chronic hepatitis B or after HBs vaccination: stimulation of the in vitro antibody response by interferon γ. Hepatology 29, 238–244 (1999).
Otten, G. et al. Enhancement of DNA vaccine potency in rhesus macaques by electroporation. Vaccine 22, 2489–2493 (2004).
Luckay, A. et al. Effect of plasmid DNA vaccine design and in vivo electroporation on the resulting vaccine-specific immune responses in rhesus macaques. J. Virol. 81, 5257–5269 (2007).
Powell, K. DNA vaccines — back in the saddle again? Nature Biotechnol. 22, 799–801 (2004).
Lorenzen, N. & LaPatra, S. E. DNA vaccines for aquacultured fish. Rev. Sci. Tech. 24, 201–213 (2005).
Norman, J. A. et al. Development of improved vectors for DNA-based immunization and other gene therapy applications. Vaccine 15, 801–803 (1997).
Moreno, S. et al. DNA immunisation with minimalistic expression constructs. Vaccine 22, 1709–1716 (2004).
Beutler, B. et al. Genetic analysis of host resistance: Toll-like receptor signaling and immunity at large. Annu. Rev. Immunol. 24, 353–389 (2006).
Klinman, D. M. Adjuvant activity of CpG oligodeoxynucleotides. Int. Rev. Immunol. 25, 135–154 (2006).
Krieg, A. M. CpG motifs in bacterial DNA and their immune effects. Annu. Rev. Immunol. 20, 709–760 (2002).
Spies, B. et al. Vaccination with plasmid DNA activates dendritic cells via Toll-like receptor 9 (TLR9) but functions in TLR9-deficient mice. J. Immunol. 171, 5908–5912 (2003). The assumption, derived largely from data on synthetic CpG oligonucleotides, that sequences in the backbone of DNA plasmid vaccines operated solely through TLR9 was challenged by the finding that DNA vaccines functioned well in Tlr−/− mice.
Rosenberg, S. A. et al. Inability to immunize patients with metastatic melanoma using plasmid DNA encoding the gp100 melanoma-melanocyte antigen. Hum. Gene Ther. 14, 709–714 (2003).
Nabel, G. J. et al. Immune response in human melanoma after transfer of an allogeneic class I major histocompatibility complex gene with DNA-liposome complexes. Proc. Natl Acad. Sci. USA 93, 15388–15393 (1996).
Tagawa, S. T. et al. Phase I study of intranodal delivery of a plasmid DNA vaccine for patients with Stage IV melanoma. Cancer 98, 144–154 (2003).
Triozzi, P. L. et al. Phase I study of a plasmid DNA vaccine encoding MART-1 in patients with resected melanoma at risk for relapse. J. Immunother. 28, 382–388 (2005).
Smith, C. L. et al. Recombinant modified vaccinia Ankara primes functionally activated CTL specific for a melanoma tumor antigen epitope in melanoma patients with a high risk of disease recurrence. Int. J. Cancer 113, 259–266 (2005). The concept that delivery of weak antigens by viral vectors will succeed has many theoretical problems. This paper illustrates that immunodomination from virus-specific CTL is one, and this is evident in a clinical trial in patients with melanoma.
Conry, R. M. et al. Safety and immunogenicity of a DNA vaccine encoding carcinoembryonic antigen and hepatitis B surface antigen in colorectal carcinoma patients. Clin. Cancer Res. 8, 2782–2787 (2002).
Mincheff, M. et al. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur. Urol. 38, 208–217 (2000).
Pavlenko, M. et al. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br. J. Cancer 91, 688–694 (2004).
Miller, A. M., Ozenci, V., Kiessling, R. & Pisa, P. Immune monitoring in a phase 1 trial of a PSA DNA vaccine in patients with hormone-refractory prostate cancer. J. Immunother. 28, 389–395 (2005).
Acknowledgements
We thank the Leukaemia Research Fund, Tenovus and Cancer Research UK for support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Jason Rice, Christian H. Ottensmeier and Freda K. Stevenson hold stock in a small company (Genvax), of which Freda K. Stevenson is also a director. Jason Rice and Freda K. Stevenson also hold patents on their DNA fusion gene vaccine designs.
Related links
Glossary
- Graft versus leukaemia
-
Following allotransplantation of bone marrow or blood stem cells from a healthy donor into an MHC-matched patient, donor T cells may recognize peptides on patient leukaemia cells that result from polymorphic differences between the two individuals, resulting in beneficial immune attack.
- Idiotypic antigen
-
Individual antigenic determinants from the variable regions of the Ig heavy and light chains are referred to as idiotopes; the sum of the individual idiotopes is referred to as the idiotype.
- Cancer-testis antigens
-
(CTA). Cancer testis antigens are encoded by germline genes but are expressed only in tumours and in male germline cells. As the latter express no MHC molecules, the CTA are effectively tumour-specific.
- Tolerance
-
The process that ensures that B- and T-cell repertoires are biased against self-reactivity, reducing the likelihood of autoimmunity.
- Regulatory T cells
-
(TReg). Regulatory CD4+ T cells serve to limit immune responses, thereby protecting against autoimmunity. There are two main types, termed natural for those developing in the thymus and adaptive for those arising in the periphery following infection and possibly cancer.
- Innate immunity
-
The first line of host defence against invading pathogenic organisms until an adaptive pathogen-specific immune response is able to develop. This is a multi-component system that includes various barriers to infection, such as physical barriers (for example, skin), physiological barriers (for example, stomach pH) and inflammatory barriers (for example, release of anti-bacterial serum proteins).
- Leader (signal) sequence
-
Leader (or signal) sequences are hydrophobic domains which are cleaved from synthesized membrane-bound or secreted proteins during transfer into the endoplasmic reticulum through the Sec61 channel. For DNA vaccines, the leader sequence is placed at the 5′ end of the encoded antigen.
- Cross-presentation
-
The uptake, processing and presentation by professional APCs of antigen that has been acquired from an exogenous source into the MHC class I pathway for induction of CD8+ T-cell responses.
- Direct presentation
-
The conventional pathway of processing and presentation of antigen into the MHC class I pathway from an endogenous intracellular source.
- Anti-CD40 antibodies
-
Agonistic anti-CD40 antibodies mimic the natural ligand (CD154) and stimulate DC to activate T-cell immunity.
- Tetanus toxin
-
This is produced by the bacterium Clostridium tetani. Treatment with formaldehyde inactivates the toxin to produce tetanus toxoid, a non-toxic but extremely immunogenic derivative that is used as a vaccine in both adults and children.
- T-cell help
-
Activated antigen-specific CD4+ T helper cells (TH) have a major role in linking and coordinating innate and adaptive immune responses. TH cells help B cells to produce antibodies and maintain circulating memory B cells; they also help stimulate and maintain both CD4+ and CD8+ T cell responses.
- Single chain Fv
-
(scFv). A recombinant polypeptide sequence consisting of the variable regions from the immunoglobulin light and heavy chains. These are usually separated by a short linker sequence to allow the variable regions to fold and assume the native conformation of the antigen binding site of the immunoglobulin from which they are derived.
- Fragment C
-
(FrC). A non-toxic yet highly immunogenic polypeptide corresponding to the ∼50 kDa carboxy-terminal portion of the heavy chain of tetanus toxin. It has a two-domain structure and the amino-terminal domain (DOM) contains a 'helper' peptide (p30) that has been shown to bind to a wide range of both murine and human MHC class II haplotypes, leading to CD4+ T-cell activation.
- 'Licensing' of dendritic cells
-
Licensing of dendritic cells by activated CD4+ T cells is essential for the generation of effective CD8+ T cell responses and possibly also for CD4+ T cell responses. Multiple interacting molecular pairs are likely to be involved, including CD40–CD40 ligand, CD28–CD80/86 and OX40–OX40 ligand.
- Immunodominant peptides
-
The natural human CTL response to an antigen tends to focus on only a few immunodominant peptides. The precise mechanisms that establish this hierarchy remain unclear but the presentation of epitopes to naive CTL is controlled at several points, including processing efficiency, capacity to bind to MHC class I molecules and the stability of the bound complex on the cell surface.
Rights and permissions
About this article
Cite this article
Rice, J., Ottensmeier, C. & Stevenson, F. DNA vaccines: precision tools for activating effective immunity against cancer. Nat Rev Cancer 8, 108–120 (2008). https://doi.org/10.1038/nrc2326
Issue Date:
DOI: https://doi.org/10.1038/nrc2326
This article is cited by
-
Current status of vaccine immunotherapy for gastrointestinal cancers
Surgery Today (2023)
-
Immunotherapy for gastroenteropancreatic neuroendocrine neoplasms (GEP-NENs): a 2021 update
Cancer Immunology, Immunotherapy (2022)
-
Considering the potential for gene-based therapy in prostate cancer
Nature Reviews Urology (2021)
-
Epigenomics and immunotherapeutic advances in pediatric brain tumors
npj Precision Oncology (2021)
-
Short microsecond pulses achieve homogeneous electroporation of elongated biological cells irrespective of their orientation in electric field
Scientific Reports (2020)