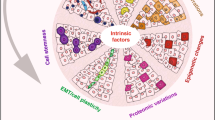
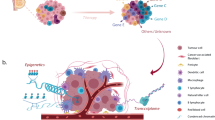
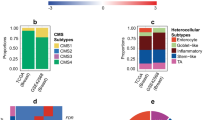
Abstract
Human breast cancers are heterogeneous, both in their pathology and in their molecular profiles. This suggests the hypothesis that breast cancers can initiate in different cell types, either breast epithelial stem cells or their progeny (transit amplifying cells or committed differentiated cells). In this respect, breast cancer could be viewed as being similar to haematological malignancies for which an analogous model has been proposed. Drawing such parallels might help to unravel the molecular nature of the initiating events in breast cancer and might have substantial clinical implications.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kinzler, K. W. & Vogelstein, B. Lessons from hereditary colorectal cancer. Cell 87, 159–170 (1996).
Deramaudt, T. & Rustgi, A. K. Mutant KRAS in the initiation of pancreatic cancer. Biochim. Biophys. Acta 1756, 97–101 (2005).
Segditsas, S. & Tomlinson, I. Colorectal cancer and genetic alterations in the Wnt pathway. Oncogene 25, 7531–7537 (2006).
Tavassoli, F. A. & Devilee, P. Pathology and Genetics of Tumours of the Breast and Female Genital Organs (International Agency for Research on Cancer, Oxford Univ. Press, Lyon, 2003).
Buerger, H. et al. Ductal invasive G2 and G3 carcinomas of the breast are the end stages of at least two different lines of genetic evolution. J. Pathol. 194, 165–170 (2001).
Buerger, H. et al. Comparative genomic hybridization of ductal carcinoma in situ of the breast-evidence of multiple genetic pathways. J. Pathol. 187, 396–402 (1999).
Buerger, H. et al. Different genetic pathways in the evolution of invasive breast cancer are associated with distinct morphological subtypes. J. Pathol. 189, 521–526 (1999).
Marsh, S. & McLeod, H. L. Pharmacogenetics and oncology treatment for breast cancer. Expert Opin. Pharmacother. 8, 119–127 (2007).
Brenton, J. D., Carey, L. A., Ahmed, A. A. & Caldas, C. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J. Clin. Oncol. 23, 7350–7360 (2005).
Sjoblom, T. et al. The consensus coding sequences of human breast and colorectal cancers. Science 314, 268–274 (2006).
Perou, C. M. et al. Molecular portraits of human breast tumours. Nature 406, 747–752 (2000).
Sorlie, T. et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc. Natl Acad. Sci. USA 98, 10869–10874 (2001).
Sorlie, T. et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl Acad. Sci. USA 100, 8418–8423 (2003).
Sotiriou, C. et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc. Natl Acad. Sci. USA 100, 10393–10398 (2003).
Bertucci, F. et al. Gene expression profiling shows medullary breast cancer is a subgroup of basal breast cancers. Cancer Res. 66, 4636–4644 (2006).
Bertucci, F. et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 65, 2170–2178 (2005).
Jones, C. et al. Expression profiling of purified normal human luminal and myoepithelial breast cells: identification of novel prognostic markers for breast cancer. Cancer Res. 64, 3037–3045 (2004).
Stingl, J., Eaves, C. J., Kuusk, U. & Emerman, J. T. Phenotypic and functional characterization in vitro of a multipotent epithelial cell present in the normal adult human breast. Differentiation 63, 201–213 (1998).
Stingl, J., Eaves, C. J., Zandieh, I. & Emerman, J. T. Characterization of bipotent mammary epithelial progenitor cells in normal adult human breast tissue. Breast Cancer Res. Treat. 67, 93–109 (2001).
Villadsen, R. et al. Evidence for a stem cell hierarchy in the adult human breast. J. Cell Biol. 177, 87–101 (2007).
Gudjonsson, T. et al. Isolation, immortalization, and characterization of a human breast epithelial cell line with stem cell properties. Genes Dev. 16, 693–706 (2002).
Dontu, G. et al. In vitro propagation and transcriptional profiling of human mammary stem/progenitor cells. Genes Dev. 17, 1253–1270 (2003).
Parmar, H. et al. A novel method for growing human breast epithelium in vivo using mouse and human mammary fibroblasts. Endocrinology 143, 4886–4896 (2002).
Stingl, J., Raouf, A., Emerman, J. T. & Eaves, C. J. Epithelial progenitors in the normal human mammary gland. J. Mammary Gland Biol. Neoplasia 10, 49–59 (2005).
Proia, D. A. & Kuperwasser, C. Reconstruction of human mammary tissues in a mouse model. Nature Protoc. 1, 206–214 (2006).
Stingl, J. et al. Purification and unique properties of mammary epithelial stem cells. Nature 439, 993–997 (2006).
Shackleton, M. et al. Generation of a functional mammary gland from a single stem cell. Nature 439, 84–88 (2006).
Smalley, M. J., Titley, J. & O'Hare, M. J. Clonal characterization of mouse mammary luminal epithelial and myoepithelial cells separated by fluorescence-activated cell sorting. In Vitro Cell Dev. Biol. Anim. 34, 711–721 (1998).
Asselin-Labat, M. L. et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nature Cell Biol. 9, 201–209 (2007).
Sleeman, K. E. et al. Dissociation of estrogen receptor expression and in vivo stem cell activity in the mammary gland. J. Cell Biol. 176, 19–26 (2007).
Asselin-Labat, M. L. et al. Steroid hormone receptor status of mouse mammary stem cells. J. Natl Cancer Inst. 98, 1011–1014 (2006).
Clarke, R. B., Howell, A., Potten, C. S. & Anderson, E. P27(KIP1) expression indicates that steroid receptor-positive cells are a non-proliferating, differentiated subpopulation of the normal human breast epithelium. Eur. J. Cancer 36 (Suppl. 4), 28–29 (2000).
Jordan, V. C. SERMs: meeting the promise of multifunctional medicines. J. Natl Cancer Inst. 99, 350–356 (2007).
Shyamala, G., Chou, Y. C., Cardiff, R. D. & Vargis, E. Effect of c-neu/ ErbB2 expression levels on estrogen receptor α-dependent proliferation in mammary epithelial cells: implications for breast cancer biology. Cancer Res. 66, 10391–10398 (2006).
Booth, B. W. & Smith, G. H. Estrogen receptor-α and progesterone receptor are expressed in label-retaining mammary epithelial cells that divide asymmetrically and retain their template DNA strands. Breast Cancer Res. 8, R49 (2006).
Cairns, J. Somatic stem cells and the kinetics of mutagenesis and carcinogenesis. Proc. Natl Acad. Sci. USA 99, 10567–10570 (2002).
Cairns, J. Mutation selection and the natural history of cancer. Nature 255, 197–200 (1975).
Abd El-Rehim, D. M. et al. High-throughput protein expression analysis using tissue microarray technology of a large well-characterised series identifies biologically distinct classes of breast cancer confirming recent cDNA expression analyses. Int. J. Cancer 116, 340–350 (2005).
Callagy, G. et al. Molecular classification of breast carcinomas using tissue microarrays. Diagn. Mol. Pathol. 12, 27–34 (2003).
Makretsov, N. A. et al. Hierarchical clustering analysis of tissue microarray immunostaining data identifies prognostically significant groups of breast carcinoma. Clin. Cancer Res. 10, 6143–6151 (2004).
Nielsen, T. O. et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 10, 5367–5374 (2004).
Naderi, A. et al. A gene-expression signature to predict survival in breast cancer across independent data sets. Oncogene 26, 1507–1516 (2007).
Teschendorff, A. E. et al. A consensus prognostic gene expression classifier for ER positive breast cancer. Genome Biol. 7, R101 (2006).
Kouros-Mehr, H., Slorach, E. M., Sternlicht, M. D. & Werb, Z. GATA-3 maintains the differentiation of the luminal cell fate in the mammary gland. Cell 127, 1041–1055 (2006).
Alizadeh, A. A. et al. Distinct types of diffuse large B-cell lymphoma identified by gene expression profiling. Nature 403, 503–511 (2000).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
O'Brien, C. A., Pollett, A., Gallinger, S. & Dick, J. E. A human colon cancer cell capable of initiating tumour growth in immunodeficient mice. Nature 445, 106–110 (2007).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 3, 730–737 (1997).
Li, C. et al. Identification of pancreatic cancer stem cells. Cancer Res. 67, 1030–1037 (2007).
Kelly, P. N., Dakic, A., Adams, J. M., Nutt, S. L. & Strasser, A. Tumor growth need not be driven by rare cancer stem cells. Science 317, 337 (2007).
Liu, R. et al. The prognostic role of a gene signature from tumorigenic breast-cancer cells. N. Engl. J. Med. 356, 217–226 (2007).
Ponti, D. et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res. 65, 5506–5511 (2005).
Neve, R. M. et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 10, 515–527 (2006).
Gordon, L. A. et al. Breast cell invasive potential relates to the myoepithelial phenotype. Int. J. Cancer 106, 8–16 (2003).
Sheridan, C. et al. CD44+/CD24− breast cancer cells exhibit enhanced invasive properties: an early step necessary for metastasis. Breast Cancer Res. 8, R59 (2006).
Hirschmann-Jax, C. et al. A distinct “side population” of cells with high drug efflux capacity in human tumor cells. Proc. Natl Acad. Sci. USA 101, 14228–14233 (2004).
Patrawala, L. et al. Side population is enriched in tumorigenic, stem-like cancer cells, whereas ABCG2+ and ABCG2− cancer cells are similarly tumorigenic. Cancer Res. 65, 6207–6219 (2005).
Cariati, N. et al. α6-Integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line. Int. J. Cancer (in the press).
Chepko, G. & Smith, G. H. Three division-competent, structurally-distinct cell populations contribute to murine mammary epithelial renewal. Tissue Cell 29, 239–253 (1997).
Zeps, N., Bentel, J. M., Papadimitriou, J. M., D'Antuono, M. F. & Dawkins, H. J. Estrogen receptor-negative epithelial cells in mouse mammary gland development and growth. Differentiation 62, 221–226 (1998).
Sapino, A., Macri, L., Gugliotta, P. & Bussolati, G. Immunocytochemical identification of proliferating cell types in mouse mammary gland. J. Histochem. Cytochem. 38, 1541–1547 (1990).
Ferguson, D. J. Ultrastructural characterisation of the proliferative (stem?) cells within the parenchyma of the normal “resting” breast. Virchows Arch. A 407, 379–385 (1985).
Ferguson, D. J. An ultrastructural study of mitosis and cytokinesis in normal 'resting' human breast. Cell Tissue Res. 252, 581–587 (1988).
Joshi, K., Smith, J. A., Perusinghe, N. & Monoghan, P. Cell proliferation in the human mammary epithelium. Differential contribution by epithelial and myoepithelial cells. Am. J. Pathol. 124, 199–206 (1986).
Preston-Martin, S., Pike, M. C., Ross, R. K., Jones, P. A. & Henderson, B. E. Increased cell division as a cause of human cancer. Cancer Res. 50, 7415–7421 (1990).
Huntly, B. J. et al. MOZ–TIF2, but not BCR–ABL, confers properties of leukemic stem cells to committed murine hematopoietic progenitors. Cancer Cell 6, 587–596 (2004).
Cozzio, A. et al. Similar MLL-associated leukemias arising from self-renewing stem cells and short-lived myeloid progenitors. Genes Dev. 17, 3029–3035 (2003).
So, C. W. et al. MLL-GAS7 transforms multipotent hematopoietic progenitors and induces mixed lineage leukemias in mice. Cancer Cell 3, 161–171 (2003).
Passegue, E., Jamieson, C. H., Ailles, L. E. & Weissman, I. L. Normal and leukemic hematopoiesis: are leukemias a stem cell disorder or a reacquisition of stem cell characteristics? Proc. Natl Acad. Sci. USA 100 (Suppl. 1), 11842–11849 (2003).
Krivtsov, A. V. et al. Transformation from committed progenitor to leukaemia stem cell initiated by MLL–AF9. Nature 442, 818–822 (2006).
Turhan, A. G. et al. Highly purified primitive hematopoietic stem cells are PML–RARA negative and generate nonclonal progenitors in acute promyelocytic leukemia. Blood 85, 2154–2161 (1995).
Dontu, G., El-Ashry, D. & Wicha, M. S. Breast cancer, stem/progenitor cells and the estrogen receptor. Trends Endocrinol. Metab. 15, 193–197 (2004).
Miyamoto, T. et al. Persistence of multipotent progenitors expressing AML1/ETO transcripts in long-term remission patients with t(8;21) acute myelogenous leukemia. Blood 87, 4789–4796 (1996).
Miyamoto, T., Weissman, I. L. & Akashi, K. AML1/ETO-expressing nonleukemic stem cells in acute myelogenous leukemia with 8;21 chromosomal translocation. Proc. Natl Acad. Sci. USA 97, 7521–7526 (2000).
Yuan, Y. et al. AML1–ETO expression is directly involved in the development of acute myeloid leukemia in the presence of additional mutations. Proc. Natl Acad. Sci. USA 98, 10398–10403 (2001).
Blair, A., Hogge, D. E., Ailles, L. E., Lansdorp, P. M. & Sutherland, H. J. Lack of expression of Thy-1 (CD90) on acute myeloid leukemia cells with long-term proliferative ability in vitro and in vivo. Blood 89, 3104–3112 (1997).
Jamieson, C. H. et al. Granulocyte-macrophage progenitors as candidate leukemic stem cells in blast-crisis CML. N. Engl. J. Med. 351, 657–667 (2004).
Shipitsin, M. et al. Molecular definition of breast tumor heterogeneity. Cancer Cell 11, 259–273 (2007).
McKenzie, J. L., Gan, O. I., Doedens, M., Wang, J. C. & Dick, J. E. Individual stem cells with highly variable proliferation and self-renewal properties comprise the human hematopoietic stem cell compartment. Nature Immunol. 7, 1225–1233 (2006).
Hope, K. J., Jin, L. & Dick, J. E. Acute myeloid leukemia originates from a hierarchy of leukemic stem cell classes that differ in self-renewal capacity. Nature Immunol. 5, 738–743 (2004).
Barabe, F., Kennedy, J. A., Hope, K. J. & Dick, J. E. Modeling the initiation and progression of human acute leukemia in mice. Science 316, 600–604 (2007).
Sleeman, K. E., Kendrick, H., Ashworth, A., Isacke, C. M. & Smalley, M. J. CD24 staining of mouse mammary gland cells defines luminal epithelial, myoepithelial/basal and non-epithelial cells. Breast Cancer Res. 8, R7 (2006).
Dimri, G., Band, H. & Band, V. Mammary epithelial cell transformation: insights from cell culture and mouse models. Breast Cancer Res. 7, 171–179 (2005).
Sun, W., Kang, K. S., Morita, I., Trosko, J. E. & Chang, C. C. High susceptibility of a human breast epithelial cell type with stem cell characteristics to telomerase activation and immortalization. Cancer Res. 59, 6118–6123 (1999).
Liu, B. Y., McDermott, S. P., Khwaja, S. S. & Alexander, C. M. The transforming activity of Wnt effectors correlates with their ability to induce the accumulation of mammary progenitor cells. Proc. Natl Acad. Sci. USA 101, 4158–4163 (2004).
Liu, S. et al. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 66, 6063–6071 (2006).
Dontu, G. et al. Role of Notch signaling in cell-fate determination of human mammary stem/progenitor cells. Breast Cancer Res. 6, R605–R615 (2004).
Li, Y. et al. Evidence that transgenes encoding components of the Wnt signaling pathway preferentially induce mammary cancers from progenitor cells. Proc. Natl Acad. Sci. USA 100, 15853–15858 (2003).
Li, Y. & Rosen, J. M. Stem/progenitor cells in mouse mammary gland development and breast cancer. J. Mammary Gland Biol. Neoplasia 10, 17–24 (2005).
Cardiff, R. D. et al. The mammary pathology of genetically engineered mice: the consensus report and recommendations from the Annapolis meeting. Oncogene 19, 968–988 (2000).
Henry, M. D., Triplett, A. A., Oh, K. B., Smith, G. H. & Wagner, K. U. Parity-induced mammary epithelial cells facilitate tumorigenesis in MMTV-neu transgenic mice. Oncogene 23, 6980–6985 (2004).
Andrechek, E. R. et al. Amplification of the neu/erbB-2 oncogene in a mouse model of mammary tumorigenesis. Proc. Natl Acad. Sci. USA 97, 3444–3449 (2000).
Abd El-Rehim, D. M. et al. Expression of luminal and basal cytokeratins in human breast carcinoma. J. Pathol. 203, 661–671 (2004).
van de Rijn, M. et al. Expression of cytokeratins 17 and 5 identifies a group of breast carcinomas with poor clinical outcome. Am. J. Pathol. 161, 1991–1996 (2002).
van 't Veer, L. J. et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature 415, 530–536 (2002).
Jumppanen, M. et al. Basal-like phenotype is not associated with patient survival in estrogen-receptor- negative breast cancers. Breast Cancer Res. 9, R16 (2007).
Yehiely, F., Moyano, J. V., Evans, J. R., Nielsen, T. O. & Cryns, V. L. Deconstructing the molecular portrait of basal-like breast cancer. Trends Mol. Med. 12, 537–544 (2006).
Livasy, C. A. et al. Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma. Mod. Pathol. 19, 264–271 (2006).
Wynford-Thomas, D. & Blaydes, J. The influence of cell context on the selection pressure for p53 mutation in human cancer. Carcinogenesis 19, 29–36 (1998).
Dumble, M. et al. The impact of altered p53 dosage on hematopoietic stem cell dynamics during aging. Blood 109, 1736–1742 (2007).
Meletis, K. et al. p53 suppresses the self-renewal of adult neural stem cells. Development 133, 363–369 (2006).
Zhao, R. et al. Analysis of p53-regulated gene expression patterns using oligonucleotide arrays. Genes Dev. 14, 981–993 (2000).
Comer, K. A. et al. Human smooth muscle α-actin gene is a transcriptional target of the p53 tumor suppressor protein. Oncogene 16, 1299–1308 (1998).
Cui, X. S. & Donehower, L. A. Differential gene expression in mouse mammary adenocarcinomas in the presence and absence of wild type p53. Oncogene 19, 5988–5996 (2000).
Foulkes, W. D. et al. Germline BRCA1 mutations and a basal epithelial phenotype in breast cancer. J. Natl Cancer Inst. 95, 1482–1485 (2003).
Elledge, S. J. & Amon, A. The BRCA1 suppressor hypothesis: an explanation for the tissue-specific tumor development in BRCA1 patients. Cancer Cell 1, 129–132 (2002).
Foulkes, W. D. BRCA1 functions as a breast stem cell regulator. J. Med. Genet. 41, 1–5 (2004).
Xu, X. et al. Conditional mutation of Brca1 in mammary epithelial cells results in blunted ductal morphogenesis and tumour formation. Nature Genet. 22, 37–43 (1999).
Cheung, A. M. et al. Brca2 deficiency does not impair mammary epithelium development but promotes mammary adenocarcinoma formation in p53+/− mutant mice. Cancer Res. 64, 1959–1965 (2004).
Jonkers, J. et al. Synergistic tumor suppressor activity of BRCA2 and p53 in a conditional mouse model for breast cancer. Nature Genet. 29, 418–425 (2001).
Wagner, K. U. et al. An adjunct mammary epithelial cell population in parous females: its role in functional adaptation and tissue renewal. Development 129, 1377–1386 (2002).
Wagner, K. U. et al. Spatial and temporal expression of the Cre gene under the control of the MMTV-LTR in different lines of transgenic mice. Transgenic Res. 10, 545–553 (2001).
Shen, Q. & Brown, P. H. Transgenic mouse models for the prevention of breast cancer. Mutat. Res. 576, 93–110 (2005).
Derksen, P. W. et al. Somatic inactivation of E-cadherin and p53 in mice leads to metastatic lobular mammary carcinoma through induction of anoikis resistance and angiogenesis. Cancer Cell 10, 437–449 (2006).
Kordon, E. C. & Smith, G. H. An entire functional mammary gland may comprise the progeny from a single cell. Development 125, 1921–1930 (1998).
Teschendorff, A. E., Naderi, A., Barbosa-Morais, N. L. & Caldas, C. PACK: profile analysis using clustering and kurtosis to find molecular classifiers in cancer. Bioinformatics 22, 2269–2275 (2006).
Teschendorff A. E., Miremadi A., Pinder S., Ellis I. O. & Caldas C. An immune response gene expression module identifies a good prognosis subtype in estrogen receptor negative breast cancer. Genome Biol. 8, R157 (2007).
Acknowledgements
J. S. is supported by funds provided by the Breast Cancer Campaign and C. C. is supported by Cancer Research UK. The authors would like to thank P. Eirew for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Rights and permissions
About this article
Cite this article
Stingl, J., Caldas, C. Molecular heterogeneity of breast carcinomas and the cancer stem cell hypothesis. Nat Rev Cancer 7, 791–799 (2007). https://doi.org/10.1038/nrc2212
Issue Date:
DOI: https://doi.org/10.1038/nrc2212
This article is cited by
-
Involving stemness factors to improve CAR T-cell-based cancer immunotherapy
Medical Oncology (2023)
-
Recursive integration of synergised graph representations of multi-omics data for cancer subtypes identification
Scientific Reports (2022)
-
Predictive and prognostic significance of BRCAness in HER2-negative breast cancer
Breast Cancer (2022)
-
Targeting CDK7 reverses tamoxifen resistance through regulating stemness in ER+ breast cancer
Pharmacological Reports (2022)
-
Hi-C profiling of cancer spheroids identifies 3D-growth-specific chromatin interactions in breast cancer endocrine resistance
Clinical Epigenetics (2021)