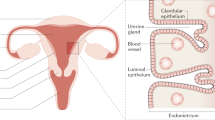
Key Points
-
Endometrial cancer is the most common gynaecological malignancy.
-
Although no specific gene or genes have been linked to the majority of cases of endometrial cancer, several well-characterized oncogenes and tumour-suppressor genes have been implicated in endometrial carcinogenesis.
-
Approximately 80% of endometrial cancer cases are type I tumours, which are usually well differentiated and endometrioid in histology, and are associated with a history of unopposed oestrogen exposure or other hyperoestrogenic risk factors such as obesity.
-
Oestrogen and selective oestrogen-receptor modulators (SERMs) are implicated in endometrial carcinogenesis through regulation of gene transcription.
-
Oestrogen and SERMs exert their carcinogenic roles in the endometrium through their downstream molecular effectors such as PAX2 (paired box gene 2).
Abstract
Endometrial cancer is the most common gynaecological cancer, and is associated with endometrial hyperplasia, unopposed oestrogen exposure and adjuvant therapy for breast cancer using selective oestrogen-receptor modulators (SERMs), particularly tamoxifen. Oestrogen and SERMs are thought to be involved in endometrial carcinogenesis through their effects on transcriptional regulation. Ultimately, oestrogen and SERMs affect the transduction of cellular signalling pathways that govern cell growth and proliferation, through downstream effectors such as PAX2 (paired box 2).
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A. et al. Cancer statistics, 2005. CA Cancer J. Clin. 55, 10–30 (2005).
Bray, F., dos Santos Silva, I., Moller, H. & Weiderpass, E. Endometrial cancer incidence trends in europe: underlying determinants and prospects for prevention. Cancer Epidemiol. Biomarkers Prev. 14, 1132–1142 (2005).
Amant, F. et al. Endometrial cancer. The Lancet 366, 491–505 (2005). A thorough and excellent discussion of current concepts about epidemiology, pathology, pathogenesis, risk factors and prevention, diagnosis, staging, prognostic factors, treatment and follow-up of endometrial cancer.
Ollikainen, M. et al. Molecular analysis of familial endometrial carcinoma: a manifestation of hereditary nonpolyposis colorectal cancer or a separate syndrome? J. Clin. Oncol. 23, 4609–4616 (2005).
Dunlop, M. et al. Cancer risk associated with germline DNA mismatch repair gene mutations. Hum. Mol. Genet. 6, 105–110 (1997).
Parc, Y. R. et al. Microsatellite instability and hMLH1/hMSH2 expression in young endometrial carcinoma patients: associations with family history and histopathology. Int. J. Cancer 86, 60–66 (2000).
Kunkel, T. A. & Erie, D. A. DNA mismatch repair. Annu. Rev. Biochem. 74, 681–710 (2005).
Marti, T. M., Kunz, C. & Fleck, O. DNA mismatch repair and mutation avoidance pathways. J. Cell Physiol. 191, 28–41 (2002).
Enomoto, T. et al. K-ras activation in premalignant and malignant epithelial lesions of the human uterus. Cancer Res. 51, 5308–5314 (1991).
Lagarda, H., Catasus, L., Arguelles, R., Matias-Guiu, X. & Prat, J. K-ras mutations in endometrial carcinomas with microsatellite instability. J. Pathol. 193, 193–199 (2001).
Lax, S. F., Kendall, B., Tashiro, H., Slebos, R. J. & Hedrick, L. The frequency of p53, K-ras mutations, and microsatellite instability differs in uterine endometrioid and serous carcinoma: evidence of distinct molecular genetic pathways. Cancer 88, 814–824 (2000).
Niederacher, D. et al. Mutations and amplification of oncogenes in endometrial cancer. Oncology 56, 59–65 (1999).
Saffari, B. et al. Amplification and overexpression of HER-2/neu (c-erbB2) in endometrial cancers: correlation with overall survival. Cancer Res. 55, 5693–5698 (1995).
Scholten, A. N., Creutzberg, C. L., van den Broek, L. J., Noordijk, E. M. & Smit, V. T. Nuclear β-catenin is a molecular feature of type I endometrial carcinoma. J. Pathol. 201, 460–465 (2003).
Moreno-Bueno, G. et al. Abnormalities of the APC/β-catenin pathway in endometrial cancer. Oncogene 21, 7981–7990 (2002).
Schlosshauer, P. W., Ellenson, L. H. & Soslow, R. A. β-Catenin and E-cadherin expression patterns in high-grade endometrial carcinoma are associated with histological subtype. Mod. Pathol. 15, 1032–1037 (2002).
Mutter, G. L. et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. J. Natl Cancer Inst. 92, 924–930 (2000).
Risinger, J. I., Hayes, A. K., Berchuck, A. & Barrett, J. C. PTEN/MMAC1 mutations in endometrial cancers. Cancer Res. 57, 4736–4738 (1997).
Obata, K. et al. Frequent PTEN/MMAC mutations in endometrioid but not serous or mucinous epithelial ovarian tumors. Cancer Res. 58, 2095–2097 (1998).
Kounelis, S. et al. Immunohistochemical profile of endometrial adenocarcinoma: a study of 61 cases and review of the literature. Mod. Pathol. 13, 379–388 (2000).
Moll, U. M., Chalas, E., Auguste, M., Meaney, D. & Chumas, J. Uterine papillary serous carcinoma evolves via a p53-driven pathway. Hum. Pathol. 27, 1295–1300 (1996).
Zheng, W., Cao, P., Zheng, M., Kramer, E. E. & Godwin, T. A. p53 overexpression and bcl-2 persistence in endometrial carcinoma: comparison of papillary serous and endometrioid subtypes. Gynecol. Oncol. 61, 167–174 (1996).
Shapiro, S. et al. Recent and past use of conjugated estrogens in relation to adenocarcinoma of the endometrium. N. Engl. J. Med. 303, 485–489 (1980).
Cole, M. P., Jones, C. T. & Todd, I. D. A new anti-oestrogenic agent in late breast cancer. An early clinical appraisal of ICI46474. Br. J. Cancer 25, 270–275 (1971).
Ward, H. W. Anti-oestrogen therapy for breast cancer: a trial of tamoxifen at two dose levels. Br. Med. J. 1, 13–14 (1973).
Fisher, B. et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J. Natl Cancer Inst. 90, 1371–1388 (1998).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Hall, J. M. & McDonnell, D. P. The estrogen receptor β-isoform (ERβ) of the human estrogen receptor modulates ERα transcriptional activity and is a key regulator of the cellular response to estrogens and antiestrogens. Endocrinology 140, 5566–5578 (1999).
McDonnell, D. P. & Norris, J. D. Connections and regulation of the human estrogen receptor. Science 296, 1642–1644 (2002). An excellent review that provides an extensive overview of the mechanistic actions of ERs.
McKenna, N. J., Lanz, R. B. & O'Malley, B. W. Nuclear receptor coregulators: cellular and molecular biology. Endocr. Rev. 20, 321–344 (1999).
Kushner, P. J. et al. Estrogen receptor pathways to AP-1. J. Steroid Biochem. Mol. Biol. 74, 311–317 (2000).
Kushner, P. J. et al. Oestrogen receptor function at classical and alternative response elements. Novartis Found. Symp. 230, 20–26 (2000).
Watanabe, T. et al. Isolation of estrogen-responsive genes with a CpG island library. Mol. Cell. Biol. 18, 442–449 (1998).
Dubik, D. & Shiu, R. P. Mechanism of estrogen activation of c-myc oncogene expression. Oncogene 7, 1587–1594 (1992).
Umayahara, Y. et al. Estrogen regulation of the insulin-like growth factor I gene transcription involves an AP-1 enhancer. J. Biol. Chem. 269, 16433–16442 (1994).
Shang, Y., Hu, X., DiRenzo, J., Lazar, M. A. & Brown, M. Cofactor dynamics and sufficiency in estrogen receptor-regulated transcription. Cell 103, 843–852 (2000).
Shang, Y. & Brown, M. Molecular determinants for the tissue specificity of SERMs. Science 295, 2465–2468 (2002).
Smith, C. L. & O'Malley, B. W. Coregulator function: a key to understanding tissue specificity of selective receptor modulators. Endocr. Rev. 25, 45–71 (2004).
Barkhem, T., Nilsson, S. & Gustafsson, J. A. Molecular mechanisms, physiological consequences and pharmacological implications of estrogen receptor action. Am. J. Pharmacogenomics 4, 19–28 (2004).
Fu, M., Wang, C., Zhang, X. & Pestell, R. Nuclear receptor modifications and endocrine cell proliferation. J. Steroid Biochem. Mol. Biol. 85, 133–138 (2003).
Wang, C. et al. Direct acetylation of the estrogen receptor α hinge region by p300 regulates transactivation and hormone sensitivity. J. Biol. Chem. 276, 18375–18383 (2001).
Wijayaratne, A. L. & McDonnell, D. P. The human estrogen receptor-α is a ubiquitinated protein whose stability is affected differentially by agonists, antagonists, and selective estrogen receptor modulators. J. Biol. Chem. 276, 35684–35692 (2001).
Tateishi, Y. et al. Ligand-dependent switching of ubiquitin-proteasome pathways for estrogen receptor. EMBO J. 23, 4813–4823 (2004).
Szego, C. M. & Davis, J. S. Adenosine 3′,5′-monophosphate in rat uterus: acute elevation by estrogen. Proc. Natl Acad. Sci. USA 58, 1711–1718 (1967).
Bjornstrom, L. & Sjoberg, M. Mechanisms of estrogen receptor signaling: convergence of genomic and nongenomic actions on target genes. Mol. Endocrinol. 19, 833–842 (2005).
Kuiper, G. G. et al. Comparison of the ligand binding specificity and transcript tissue distribution of estrogen receptors α and β. Endocrinology 138, 863–870 (1997).
Lecce, G., Meduri, G., Ancelin, M., Bergeron, C. & Perrot-Applanat, M. Presence of estrogen receptor β in the human endometrium through the cycle: expression in glandular, stromal, and vascular cells. J. Clin. Endocrinol. Metab. 86, 1379–1386 (2001).
Weihua, Z. et al. Estrogen receptor (ER) β, a modulator of ERα in the uterus. Proc. Natl Acad. Sci. USA 97, 5936–5941 (2000).
Jazaeri, A. A. et al. Well-differentiated endometrial adenocarcinomas and poorly differentiated mixed mullerian tumors have altered ER and PR isoform expression. Oncogene 20, 6965–6969 (2001).
Saegusa, M. & Okayasu, I. Changes in expression of estrogen receptors α and β in relation to progesterone receptor and pS2 status in normal and malignant endometrium. Jpn J. Cancer Res. 91, 510–518 (2000).
Horvath, G., Leser, G., Hahlin, M. & Henriksson, M. Exon deletions and variants of human estrogen receptor mRNA in endometrial hyperplasia and adenocarcinoma. Int. J. Gynecol. Cancer 10, 128–136 (2000).
Bryant, W., Snowhite, A. E., Rice, L. W. & Shupnik, M. A. The estrogen receptor (ER)α variant δ5 exhibits dominant positive activity on ER-regulated promoters in endometrial carcinoma cells. Endocrinology 146, 751–759 (2005).
Herynk, M. H. & Fuqua, S. A. Estrogen receptor mutations in human disease. Endocr. Rev. 25, 869–898 (2004).
Ogawa, S. et al. Molecular cloning and characterization of human estrogen receptor βcx: a potential inhibitor ofestrogen action in human. Nucleic Acids Res. 26, 3505–3512 (1998).
Saji, S. et al. Expression of estrogen receptor (ER) (β)cx protein in ER(α)-positive breast cancer: specific correlation with progesterone receptor. Cancer Res. 62, 4849–4853 (2002).
Critchley, H. O. et al. Wild-type estrogen receptor (ERβ1) and the splice variant (ERβcx/β2) are both expressed within the human endometrium throughout the normal menstrual cycle. J. Clin. Endocrinol. Metab. 87, 5265–5273 (2002).
Skrzypczak, M. et al. Evaluation of mRNA expression of estrogen receptor β and its isoforms in human normal and neoplastic endometrium. Int. J. Cancer 110, 783–787 (2004).
Goldstein, S. R. The effect of SERMs on the endometrium. Ann. NY Acad. Sci. 949, 237–242 (2001).
Jordan, V. C., Gapstur, S. & Morrow, M. Selective estrogen receptor modulation and reduction in risk of breast cancer, osteoporosis, and coronary heart disease. J. Natl Cancer Inst. 93, 1449–1457 (2001).
Jordan, V. C. Is tamoxifen the Rosetta stone for breast cancer? J. Natl Cancer Inst. 95, 338–340 (2003).
Jordan, V. C. Tamoxifen: a most unlikely pioneering medicine. Nature Rev. Drug Discov. 2, 205–213 (2003).
Jordan, V. C. Selective estrogen receptor modulation: concept and consequences in cancer. Cancer Cell 5, 207–213 (2004). As a pioneer in the field of SERM research and application, the lead author in references 59–62 discusses the historical perspective, tissue specificity, mechanistic actions and clinical implications of SERMs.
Smith, R. E. & Good, B. C. Chemoprevention of breast cancer and the trials of the National Surgical Adjuvant Breast and Bowel Project and others. Endocr. Relat. Cancer 10, 347–357 (2003).
Goldstein, S. R. et al. A 12-month comparative study of raloxifene, estrogen, and placebo on the postmenopausal endometrium. Obstet. Gynecol. 95, 95–103 (2000).
Sporn, M. B. Arzoxifene: a promising new selective estrogen receptor modulator for clinical chemoprevention of breast cancer. Clin. Cancer Res. 10, 5313–5315 (2004).
Martel, C. et al. Prevention of bone loss by EM-800 and raloxifene in the ovariectomized rat. J. Steroid Biochem. Mol. Biol. 74, 45–56 (2000).
Willson, T. M. et al. 3-[4-(1, 2-Diphenylbut-1-enyl)phenyl]acrylic acid: a non-steroidal estrogen with functional selectivity for bone over uterus in rats. J. Med. Chem. 37, 1550–1552 (1994).
Simoncini, T. et al. Genomic and nongenomic mechanisms of nitric oxide synthesis induction in human endothelial cells by a fourth-generation selective estrogen receptor modulator. Endocrinology 143, 2052–2061 (2002).
Brady, H. et al. Effects of SP500263, a novel, potent antiestrogen, on breast cancer cells and in xenograft models. Cancer Res. 62, 1439–1442 (2002).
Shiau, A. K. et al. The structural basis of estrogen receptor/coactivator recognition and the antagonism of this interaction by tamoxifen. Cell 95, 927–937 (1998).
Brzozowski, A. M. et al. Molecular basis of agonism and antagonism in the oestrogen receptor. Nature 389, 753–758 (1997).
Tanenbaum, D. M., Wang, Y., Williams, S. P. & Sigler, P. B. Crystallographic comparison of the estrogen and progesterone receptor's ligand binding domains. Proc. Natl Acad. Sci. USA 95, 5998–6003 (1998).
Pike, A. C. et al. Structural insights into the mode of action of a pure antiestrogen. Structure 9, 145–153 (2001).
Jordan, V. C. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 1. Receptor interactions. J. Med. Chem. 46, 883–908 (2003).
Jordan, V. C. Antiestrogens and selective estrogen receptor modulators as multifunctional medicines. 2. Clinical considerations and new agents. J. Med. Chem. 46, 1081–1111 (2003).
Paige, L. A. et al. Estrogen receptor (ER) modulators each induce distinct conformational changes in ER α and ER β. Proc. Natl Acad. Sci. USA 96, 3999–4004 (1999).
McDonnell, D. P. Mining the complexities of the estrogen signaling pathways for novel therapeutics. Endocrinology 144, 4237–4240 (2003).
McDonnell, D. P. The molecular pharmacology of SERMs. Trends Endocrinol. Metab. 10, 301–311 (1999).
Carmichael, P. L. et al. Lack of evidence from HPLC 32P-post-labelling for tamoxifen–DNA adducts in the human endometrium. Carcinogenesis 20, 339–342 (1999).
Beland, F. A., McDaniel, L. P. & Marques, M. M. Comparison of the DNA adducts formed by tamoxifen and 4-hydroxytamoxifen in vivo. Carcinogenesis 20, 471–477 (1999).
Bartsch, H. et al. Lack of evidence for tamoxifen- and toremifene-DNA adducts in lymphocytes of treated patients. Carcinogenesis 21, 845–847 (2000).
Shibutani, S. et al. Identification of tamoxifen-DNA adducts in the endometrium of women treated with tamoxifen. Carcinogenesis 21, 1461–1467 (2000).
Phillips, D. H. Understanding the genotoxicity of tamoxifen? Carcinogenesis 22, 839–849 (2001).
Schild, L. J. et al. Formation of tamoxifen–DNA adducts in multiple organs of adult female cynomolgus monkeys dosed with tamoxifen for 30 days. Cancer Res. 63, 5999–6003 (2003).
Shibutani, S. et al. Identification of tamoxifen-DNA adducts in monkeys treated with tamoxifen. Cancer Res. 63, 4402–4406 (2003).
Watanabe, T. et al. Agonistic effect of tamoxifen is dependent on cell type, ERE-promoter context, and estrogen receptor subtype: functional difference between estrogen receptors α and β. Biochem. Biophys. Res. Commun. 236, 140–145 (1997).
Robertson, J. A., Bhattacharyya, S. & Ing, N. H. Tamoxifen up-regulates oestrogen receptor-α, c-fos and glyceraldehyde 3-phosphate-dehydrogenase mRNAs in ovine endometrium. J. Steroid Biochem. Mol. Biol. 67, 285–292 (1998).
Jones, P. S., Parrott, E. & White, I. N. Activation of transcription by estrogen receptor α and β is cell type- and promoter-dependent. J. Biol. Chem. 274, 32008–32014 (1999).
Russo, L. A., Calabro, S. P., Filler, T. A., Carey, D. J. & Gardner, R. M. In vivo regulation of syndecan-3 expression in the rat uterus by 17 β-estradiol. J. Biol. Chem. 276, 686–692 (2001).
Dardes, R. C. et al. Regulation of estrogen target genes and growth by selective estrogen-receptor modulators in endometrial cancer cells. Gynecol. Oncol. 85, 498–506 (2002).
Wang, Z. et al. Tamoxifen regulates human telomerase reverse transcriptase (hTERT) gene expression differently in breast and endometrial cancer cells. Oncogene 21, 3517–3524 (2002).
Hague, S. et al. Tamoxifen induction of angiogenic factor expression in endometrium. Br. J. Cancer 86, 761–767 (2002).
Sakamoto, T. et al. Estrogen receptor-mediated effects of tamoxifen on human endometrial cancer cells. Mol. Cell. Endocrinol. 192, 93–104 (2002).
Castro-Rivera, E. & Safe, S. 17 β-estradiol- and 4-hydroxytamoxifen-induced transactivation in breast, endometrial and liver cancer cells is dependent on ER-subtype, cell and promoter context. J. Steroid Biochem. Mol. Biol. 84, 23–31 (2003).
Bramlett, K. S. & Burris, T. P. Target specificity of selective estrogen receptor modulators within human endometrial cancer cells. J. Steroid Biochem. Mol. Biol. 86, 27–34 (2003).
Paech, K. et al. Differential ligand activation of estrogen receptors ERα and ERβ at AP1 sites. Science 277, 1508–1510 (1997).
Webb, P., Nguyen, P. & Kushner, P. J. Differential SERM effects on corepressor binding dictate ERα activity in vivo. J. Biol. Chem. 278, 6912–6920 (2003).
Klotz, D. M., Hewitt, S. C., Korach, K. S. & Diaugustine, R. P. Activation of a uterine insulin-like growth factor I signaling pathway by clinical and environmental estrogens: requirement of estrogen receptor-α. Endocrinology 141, 3430–3439 (2000).
Jepsen, K. et al. Combinatorial roles of the nuclear receptor corepressor in transcription and development. Cell 102, 753–763 (2000).
Katzenellenbogen, B. S. et al. Antiestrogens: mechanisms and actions in target cells. J. Steroid Biochem. Mol. Biol. 53, 387–393 (1995).
Montano, M. M., Muller, V., Trobaugh, A. & Katzenellenbogen, B. S. The carboxy-terminal F domain of the human estrogen receptor: role in the transcriptional activity of the receptor and the effectiveness of antiestrogens as estrogen antagonists. Mol. Endocrinol. 9, 814–825 (1995).
McInerney, E. M., Tsai, M. J., O'Malley, B. W. & Katzenellenbogen, B. S. Analysis of estrogen receptor transcriptional enhancement by a nuclear hormone receptor coactivator. Proc. Natl Acad. Sci. USA 93, 10069–10073 (1996).
Smith, C. L., Nawaz, Z. & O'Malley, B. W. Coactivator and corepressor regulation of the agonist/antagonist activity of the mixed antiestrogen, 4-hydroxytamoxifen. Mol. Endocrinol. 11, 657–666 (1997).
Webb, P. et al. Estrogen receptor activation function 1 works by binding p160 coactivator proteins. Mol. Endocrinol. 12, 1605–1618 (1998).
Jackson, T. A. et al. The partial agonist activity of antagonist-occupied steroid receptors is controlled by a novel hinge domain-binding coactivator L7/SPA and the corepressors N-CoR or SMRT. Mol. Endocrinol. 11, 693–705 (1997).
Wu, H. et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature 438, 981–987 (2005).
Pole, J., Carmichael, P. & Griffin, J. Identification of transcriptional biomarkers induced by SERMS in human endometrial cells using multivariate analysis of DNA microarrays. Biomarkers 9, 447–460 (2004).
Pole, J. C., Gold, L. I., Orton, T., Huby, R. & Carmichael, P. L. Gene expression changes induced by estrogen and selective estrogen receptor modulators in primary-cultured human endometrial cells: signals that distinguish the human carcinogen tamoxifen. Toxicology 206, 91–109 (2005).
Farnell, Y. Z. & Ing, N. H. Endometrial effects of selective estrogen receptor modulators (SERMs) on estradiol-responsive gene expression are gene and cell-specific. J. Steroid Biochem. Mol. Biol. 84, 513–526 (2003).
Saville, B. et al. Ligand-, cell-, and estrogen receptor subtype (α/β)-dependent activation at GC-rich (Sp1) promoter elements. J. Biol. Chem. 275, 5379–5387 (2000).
Fournier, B. et al. Estrogen receptor (ER)-α, but not ER-β, mediates regulation of the insulin-like growth factor I gene by antiestrogens. J. Biol. Chem. 276, 35444–35449 (2001).
Berry, M., Metzger, D. & Chambon, P. Role of the two activating domains of the oestrogen receptor in the cell-type and promoter-context dependent agonistic activity of the anti-oestrogen 4-hydroxytamoxifen. EMBO J. 9, 2811–2818 (1990).
Metzger, D., Losson, R., Bornert, J. M., Lemoine, Y. & Chambon, P. Promoter specificity of the two transcriptional activation functions of the human oestrogen receptor in yeast. Nucleic Acids Res. 20, 2813–2817 (1992).
Tzukerman, M. T. et al. Human estrogen receptor transactivational capacity is determined by both cellular and promoter context and mediated by two functionally distinct intramolecular regions. Mol. Endocrinol. 8, 21–30 (1994).
Pham, T. A., Hwung, Y. P., Santiso, D., McDonnell, D. P. & O'Malley, B. W. Ligand-dependent and -independent function of the transactivation regions of the human estrogen receptor in yeast. Mol. Endocrinol. 6, 1043–1050 (1992).
Mansouri, A., Goudreau, G. & Gruss, P. Pax genes and their role in organogenesis. Cancer Res. 59, 1707s–1709s (1999).
Chi, N. & Epstein, J. A. Getting your Pax straight: Pax proteins in development and disease. Trends Genet. 18, 41–47 (2002).
Stuart, E. T. & Gruss, P. PAX: developmental control genes in cell growth and differentiation. Cell Growth Differ. 7, 405–412 (1996).
Hutson, M. R., Lewis, J. E., Nguyen-Luu, D., Lindberg, K. H. & Barald, K. F. Expression of Pax2 and patterning of the chick inner ear. J. Neurocytol. 28, 795–807 (1999).
Joly, D. et al. Pax2 in the development of renal and urinary tract diseases. Adv. Nephrol. Necker Hosp. 29, 317–327 (1999).
Eccles, M. R. et al. PAX genes in development and disease: the role of PAX2 in urogenital tract development. Int. J. Dev. Biol. 46, 535–544 (2002).
Torres, M., Gomez-Pardo, E., Dressler, G. R. & Gruss, P. Pax-2 controls multiple steps of urogenital development. Development 121, 4057–4065 (1995).
Torres, M., Gomez-Pardo, E. & Gruss, P. Pax2 contributes to inner ear patterning and optic nerve trajectory. Development 122, 3381–3391 (1996).
Favor, J. et al. The mouse Pax2(1Neu) mutation is identical to a human PAX2 mutation in a family with renal-coloboma syndrome and results in developmental defects of the brain, ear, eye, and kidney. Proc. Natl Acad. Sci. USA 93, 13870–13875 (1996).
Kroll, T. G. et al. PAX8–PPARγ1 fusion oncogene in human thyroid carcinoma [corrected]. Science 289, 1357–1360 (2000).
Cazzaniga, G. et al. The paired box domain gene PAX5 is fused to ETV6/TEL in an acute lymphoblastic leukemia case. Cancer Res. 61, 4666–4670 (2001).
Murer, L. et al. Expression of nuclear transcription factor PAX2 in renal biopsies of juvenile nephronophthisis. Nephron 91, 588–593 (2002).
Marques, A. R. et al. Expression of PAX8-PPAR γ 1 rearrangements in both follicular thyroid carcinomas and adenomas. J. Clin. Endocrinol. Metab. 87, 3947–3952 (2002).
Robson, E. J., He, S. J. & Eccles, M. R. A PANorama of PAX genes in cancer and development. Nature Rev. Cancer 6, 52–62 (2006). A thoughtful and up-to-date review of the oncogenic roles of Pax genes.
Racz, A. et al. Gene amplification at chromosome 1pter-p33 including the genes PAX7 and ENO1 in squamous cell lung carcinoma. Int. J. Oncol. 17, 67–73 (2000).
Barr, F. G. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene 20, 5736–5746 (2001).
Nishimoto, K. et al. PAX2 gene mutation in a family with isolated renal hypoplasia. J. Am. Soc. Nephrol. 12, 1769–1772 (2001).
Salomon, R. et al. PAX2 mutations in oligomeganephronia. Kidney Int. 59, 457–462 (2001).
Scholl, F. A. et al. PAX3 is expressed in human melanomas and contributes to tumor cell survival. Cancer Res. 61, 823–826 (2001).
Muratovska, A., Zhou, C., He, S., Goodyer, P. & Eccles, M. R. Paired-Box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene 22, 7989–7997 (2003).
Maulbecker, C. C. & Gruss, P. The oncogenic potential of Pax genes. EMBO J. 12, 2361–2367 (1993).
Stuart, E. T. & Gruss, P. PAX genes: what's new in developmental biology and cancer? Hum. Mol. Genet. 4, s1717–s1720 (1995).
Silberstein, G. B., Dressler, G. R. & Van Horn, K. Expression of the PAX2 oncogene in human breast cancer and its role in progesterone-dependent mammary growth. Oncogene 21, 1009–1016 (2002).
Khoubehi, B. et al. Expression of the developmental and oncogenic PAX2 gene in human prostate cancer. J. Urol. 165, 2115–2120 (2001).
Dressler, G. R. & Douglass, E. C. Pax-2 is a DNA-binding protein expressed in embryonic kidney and Wilms tumor. Proc. Natl Acad. Sci. USA 89, 1179–1183 (1992).
Eccles, M. R. et al. Expression of the PAX2 gene in human fetal kidney and Wilms' tumor. Cell Growth Differ. 3, 279–289 (1992).
Eccles, M. R., Yun, K., Reeve, A. E. & Fidler, A. E. Comparative in situ hybridization analysis of PAX2, PAX8, and WT1 gene transcription in human fetal kidney and Wilms' tumors. Am. J. Pathol. 146, 40–45 (1995).
Cho, K. S., Elizondo, L. I. & Boerkoel, C. F. Advances in chromatin remodeling and human disease. Curr. Opin. Genet. Dev. 14, 308–315 (2004).
Brophy, P. D., Ostrom, L., Lang, K. M. & Dressler, G. R. Regulation of ureteric bud outgrowth by Pax2-dependent activation of the glial derived neurotrophic factor gene. Development 128, 4747–4756 (2001).
McConnell, M. J., Cunliffe, H. E., Chua, L. J., Ward, T. A. & Eccles, M. R. Differential regulation of the human Wilms tumour suppressor gene (WT1) promoter by two isoforms of PAX2. Oncogene 14, 2689–2700 (1997).
Dehbi, M., Ghahremani, M., Lechner, M., Dressler, G. & Pelletier, J. The paired-box transcription factor, PAX2, positively modulates expression of the Wilms' tumor suppressor gene (WT1). Oncogene 13, 447–453 (1996).
Brophy, P. D., Lang, K. M. & Dressler, G. R. The secreted frizzled related protein 2 (SFRP2) gene is a target of the Pax2 transcription factor. J. Biol. Chem. 278, 52401–5245 (2003).
Cummings, S. R. et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. JAMA 281, 2189–2197 (1999).
Risinger, J. I., Maxwell, G. L., Berchuck, A. & Barrett, J. C. Promoter hypermethylation as an epigenetic component in Type I and Type II endometrial cancers. Ann. NY Acad. Sci. 983, 208–212 (2003).
Acknowledgements
Work in laboratory of Y.S. was supported by grants from the National Natural Science Foundation of China and by the '863 Program' and the '973 Program' of the Ministry of Science and Technology of China.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Related links
Related links
DATABASES
National Cancer Institute
FURTHER INFORMATION
Breast Cancer Prevention Trial
National Surgical Adjuvant Breast and Bowel Project
The Study of Tamoxifen and Raloxifene
United States National Toxicology Program listed steroidal oestrogens as carcinogens (2002)
Glossary
- Endometrioid
-
A tumour containing epithelial or stromal elements resembling endometrial tissue.
- SERMs
-
A group of chemical compounds that are structurally related to oestrogens, biochemically bind to oestrogen receptors, and functionally exert different effects on different tissues.
- Second-, third- and fourth-generation SERMs
-
Chemical compounds that are developed in different stages and are intended to improve the beneficial effects and reduce the harmful effects of original SERMs.
- Genotoxicity
-
Effects that cause genetic mutations and/or changes in chromosome structure and number.
- Canonical
-
Standard or well-established and recognized DNA sequences to which transcription factors bind.
Rights and permissions
About this article
Cite this article
Shang, Y. Molecular mechanisms of oestrogen and SERMs in endometrial carcinogenesis. Nat Rev Cancer 6, 360–368 (2006). https://doi.org/10.1038/nrc1879
Issue Date:
DOI: https://doi.org/10.1038/nrc1879
This article is cited by
-
2′,3′,4′-Trihydroxychalcone changes estrogen receptor α regulation of genes and breast cancer cell proliferation by a reprogramming mechanism
Molecular Medicine (2022)
-
High Maternal Serum Estradiol in First Trimester of Multiple Pregnancy Contributes to Small for Gestational Age via DNMT1-Mediated CDKN1C Upregulation
Reproductive Sciences (2022)
-
GNA14 stimulation of KLF7 promotes malignant growth of endometrial cancer through upregulation of HAS2
BMC Cancer (2021)
-
IL-37bΔ1-45 suppresses the migration and invasion of endometrial cancer cells by targeting the Rac1/NF-κB/MMP2 signal pathway
Laboratory Investigation (2021)
-
Estrogen enhances the proliferation and migration of ovarian cancer cells by activating transient receptor potential channel C3
Journal of Ovarian Research (2020)