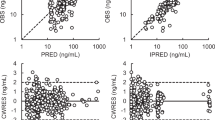
Key Points
-
The field of pharmacokinetics attempts to describe the bodily disposition of drugs in terms of numerical parameters that are calculated from plasma concentrations obtained at time points after drug administration. These parameters can be correlated with treatment outcomes such as toxicity and efficacy.
-
Anticancer drugs can have considerable interindividual pharmacokinetic variability, which, given the narrow therapeutic index of these drugs, results in unpredictable clinical effects.
-
There are many potential sources of pharmacokinetic variation, including interindividual differences in absorption, distribution, metabolism and excretion of anticancer drugs.
-
A considerable amount of research is currently directed towards defining genetic polymorphisms in drug-disposition pathways that explain observed interindividual pharmacokinetic variability.
-
Tailoring doses of anticancer drug to the individual patient will decrease interpatient pharmacokinetic variability and reduce the risks of severe toxicity and subtherapeutic treatment.
-
Individualized dosing strategies currently being examined in the research setting are promising, although few are used in routine clinical practice.
Abstract
The translation of advances in cancer biology to drug discovery can be complicated by pharmacokinetic variation between individuals and within individuals, and this can result in unpredictable toxicity and variable antineoplastic effects. Previously unrecognized variables (such as genetic polymorphisms) are now known to have a significant impact on drug disposition. How can the pharmacokinetic variability of anticancer agents be reduced? This will require the understanding of correlations between pharmacokinetics and treatment outcomes, the identification of relevant patient parameters, mathematical modelling of individual and population pharmacokinetics, and the development of algorithms that will tailor doses to the individual patient.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Evans, W. E. & Relling, M. V. Clinical pharmacokinetics–pharmacodynamics of anticancer drugs. Clin. Pharmacokinet. 16, 327–336 (1989).
Freyer, G. et al. Pharmacokinetic studies in cancer chemotherapy: usefulness in clinical practice. Cancer Treat. Rev. 23, 153–169 (1997).
Masson, E. & Zamboni, W. C. Pharmacokinetic optimisation of cancer chemotherapy: effect on outcomes. Clin. Pharmacokinet. 32, 324–343 (1997).
Baker, S. D. et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991–2001. J. Natl Cancer Inst. 94, 1883–1888 (2002). In this retrospective analysis of the pharmacokinetics of 33 investigational agents as a function of BSA, BSA-based dosing was only associated with a reduction in interindividual variability in drug clearance for five agents.
Hande, K., Messenger, M., Wagner, J., Krozely, M. & Kaul, S. Inter- and intraindividual variability in etoposide kinetics with oral and intravenous drug administration. Clin. Cancer Res. 5, 2742–2747 (1999).
Carreca, I. & Balducci, L. Oral chemotherapy of cancer in the elderly. Am. J. Cancer 1, 101–108 (2002).
Demario, M. D. & Ratain, M. J. Oral chemotherapy: rational and future directions. J. Clin. Oncol. 17, 2557–2567 (1998).
Juliano, R. L. & Ling, V. A. A surface glycoprotein modulating drug permeability in Chinese hamster ovary cell mutants. Biochem. Biophys. Acta. 455, 152–162 (1976).
Riordan, J. R. et al. Amplification of P-glycoprotein genes in multidrug-resistant mammalian cell lines. Nature 316, 817–819 (1985).
Kartner, N., Evernden-Porelle, D., Bradley, G. & Ling, V. Detection of P-glycoprotein in multidrug-resistant cell lines by monoclonal antibodies. Nature 316, 820–823 (1985).
Roninson, I. B. et al. Isolation of human mdr DNA sequences amplified in multidrug-resistant KB carcinoma cells. Proc. Natl Acad. Sci. USA 83, 4538–4542 (1986).
Higgins, C. F. ABC transporters: from microorganisms to man. Annu. Rev. Cell Biol. 8, 67–113 (1992).
Sugawara, I. et al. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by monoclonal antibody, MRK16. Cancer Res. 48, 1926–1929 (1988).
Thiebaut, F. et al. Cellular localization of the multidrug resistance gene product in normal human tissues. Proc. Natl Acad. Sci. USA 84, 7735–7738 (1987).
Sparreboom, A. et al. Limited oral bioavailability and active epithelial excretion of paclitaxel caused by P-glycoprotein in the intestine. Proc. Natl Acad. Sci USA 94, 2031–2035 (1997).
Bardelmeijer, H. A., van Tellingen, O., Schellens, J. H. M. & Beijnen, J. H. The oral route for the administration of cytotoxic drugs: strategies to increase the efficiency and consistency of drug delivery. Invest. New Drugs 18, 231–241 (2000).
van Asperen, J. et al. Enhanced oral bioavailability of paclitaxel in mice treated with the P-glycoprotein blocker SDZ PSC 833. Br. J. Cancer 76, 1181–1183 (1997).
Bardelmeijer, H. A. et al. Increased oral bioavailability of paclitaxel by GF120918 in mice through selective modulation of P-glycoprotein. Clin. Cancer Res. 6, 4416–4421 (2000).
Woo, J. S., Lee, C. H., Shim, C. K. & Hwang, S. J. Enhanced oral bioavailability of paclitaxel by coadministration of the P-glycoprotein inhibitor KR30031. Pharm. Res. 20, 24–30 (2003).
Bardelmeijer, H. A., Ouwehand, M., Beijnen, J. H., Schellens, J. H. & van Tellingen, O. Efficacy of novel P-glycoprotein inhibitors to increase the oral uptake of paclitaxel in mice. Invest. New Drugs 22, 219–229 (2004).
Hoffmeyer, S. et al. Functional polymorphisms of the human multi-drug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc. Natl Acad. Sci. USA 97, 3473–3478 (2000).
Hitzl, M. et al. The C3435T mutation in the human MDR1 gene is associated with altered efflux of the P-glycoprotein substrate rhodamine 123 from CD56+ natural killer cells. Pharmacogenetics 11, 293–298 (2001).
Kerb, R. et al. The predictive value of MDR1, CYP2C9, and CYP2C19 polymorphisms for phenytoin plasma levels. Pharmacogenom. J. 1, 204–210 (2001).
Kerb, R., Hoffmeyer, S. & Brinkmann, U. ABC drug transporters: hereditary polymorphisms and pharmacological impact in MDR1, MRP1 and MRP2. Pharmacogenomics 2, 51–64 (2001).
Cascorbi, I. et al. Frequency of single nucleotide polymorphisms in the P-glycoprotein drug transporter MDR1 gene in white subjects. Clin. Pharmacol. Ther. 69, 169–174 (2001).
Kurata, Y. et al. Role of human MDR1 gene polymorphism in bioavailability and interaction of digoxin, a substrate of P-glycoprotein. Clin. Pharmacol. Ther. 72, 209–219 (2002).
Fromm, M. F. et al. The effect of rifampin treatment on intestinal expression of human MRP transporters. Am. J. Pathol. 157, 1575–1580 (2000).
Zamber, C. P. et al. Natural allelic variants of breast cancer resistant protein (BCRP) and their relationship to BCRP expression in human intestine. Pharmacogenetics 13, 19–28 (2003).
Watkins, P. B., Wrighton, S. A., Schuetz, E. G., Molowa, D. T. & Guzelian, P. S. Identification of glucocorticoid-inducible cytochromes P-450 in the intestinal mucosa of rats and man. J. Clin. Invest. 80, 1029–1036 (1987).
Kolars, J. C., Schmiedlin-Ren, P., Schuetz, J. D., Fang, C. & Watkins, P. B. Identification of rifampin-inducible P450IIIA4 (CYP3A4) in human small bowel enterocytes. J. Clin. Invest. 90, 1871–1888 (1992).
Murray, G. I. et al. The immunocytochemical localization and distribution of cytochrome P-450 in normal human hepatic and extrahepatic tissues with a monoclonal antibody to human cytochrome P-450. Br. J. Clin. Pharmacol. 25, 465–475 (1988).
de Waziers, P. H., Cugnenc, P. H., Yang, C. S., Leroux, J. P. & Beaune, P. H. Cytochrome P450 isoenzymes, epoxide hydrolase and glutathione transferases in rat and human hepatic and extrahepatic tissues. J. Pharmacol. Exp. Ther. 253, 387–394 (1990).
Peters, W. H. & Kremers, P. G. Cytochromes P-450 in the intestinal mucosa of man. Biochem. Pharmacol. 38, 1535–1538 (1989).
Kolars, J. C. et al. CYP3A gene expression in human gut epithelium. Pharmacogenetics 4, 247–259 (1994).
McKinnon, R. A. et al. Characterization of CYP3A gene subfamily expression in human gastrointestinal tissues. Gut 36, 259–267 (1995).
Kolars, J. C., Awni, W. M., Merion, R. M. & Watkins, P. B. First-pass metabolism of cyclosporin by the gut. Lancet 338, 1488–1490 (1991). By instilling cyclosporine into the small bowel of patients during the anhepatic phase of liver transplantation and measuring cyclosporine metabolites, the authors demonstrated that cyclosporine is metabolized in the intestinal wall.
Lown, K. S. et al. Interindividual heterogeneity in expression of CYP3A4 and CYP3A5 in small bowel. Lack of prediction by the erythromycin breath test. Drug Metab. Dispos. 22, 947–955 (1994).
Zhang, Q. Y. et al. Characterization of human small intestinal cytochromes P-450. Drug Metab. Dispos. 27, 804–809 (1999).
Vree, T. B. et al. Decreased plasma albumin concentration results in increased volume of distribution and decreased elimination of midazolam in intensive care patients. Clin. Pharmacol. Ther. 46, 537–544 (1989).
Blair, E. Y., Rivory, L. P., Clarke, S. J. & McLachlan, A. J. Population pharmacokinetics of ralitrexed in patients with advanced solid tumours. Br. J. Clin. Pharmacol. 57, 416–426 (2004).
Sparreboom, A. et al. Effects of α1-acid glycoprotein on the clinical pharmacokinetics of 7-hydroxystaurosporine. Clin. Cancer Res. 10, 6840–6846 (2004).
Slaviero, K. A., Clarke, S. J. & Rivory, L. P. Inflammatory response: an unrecognised source of variability in the pharmacokinetics and pharmacodynamics of cancer chemotherapy. Lancet Oncol. 4, 224–232 (2003).
Marzolini, C., Tirona, R. G. & Kim, R. B. Pharmacogenomics of the OATP and OAT families. Pharmacogenomics 5, 273–282 (2004).
Nishizato, Y. et al. Polymorphisms of OATP-C (SLC21A6) and OAT3 (SLC22A8) genes: consequences for pravastatin pharmacokinetics. Clin. Pharmacol. Ther. 73, 554–565 (2003).
Kivistö, K. T., Kroemer, H. K. & Eichelbaum, M. The role of human cytochrome P450 enzymes in the metabolism of anticancer agents: implications for drug interactions. Br. J. Clin. Pharmacol. 40, 523–530 (1995).
Li, A. P., Kaminski, D. L. & Rasmussen, A. Substrates of human hepatic cytochrome P450 3A4. Toxicology 104, 1–8 (1995).
Evans, W. E. & Relling, M. V. Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286, 487–491 (1999).
Wrighton, S. A. et al. Studies on the expression and metabolic capabilities of human liver cytochrome P450IIIA5 (HLp3). Mol. Pharmacol. 38, 207–213 (1990).
Shimada, T., Yamazaki, H., Mimura, M., Inui, Y. & Guengerich, F. P. Interindividual variations in human liver cytochrome P-450 enzymes involved in the oxidation of drugs, carcinogens, and toxic chemicals: studies with liver microsomes of 30 Japanese and 30 Caucasians. J. Pharmacol. Exp. Ther. 270, 414–423 (1994).
Tomonori, T. et al. No ethnic difference between Caucasian and Japanese hepatic samples in the expression frequency of CYP3A5 and CYP3A7. Biochem. Pharmacol. 57, 935–939 (1999).
Williams, M. L. et al. A discordance of the cytochrome P450 2C19 genotype and phenotype in patients with advanced cancer. Br. J. Clin. Pharmacol. 49, 485–488 (2000).
Baker, S. D. et al. Factors affecting cytochrome P-450 3A activity in cancer patients. Clin. Cancer Res. 10, 8341–8350 (2004).
Toomey, D., Redmond, H. P. & Bouchier-Hayes, D. Mechanisms mediating cancer cachexia. Cancer 76, 2418–2426 (1995).
Mantovani, G. et al. Cytokine activity in cancer-related anorexia/cachexia; role of megestrol acetate and medroxyprogesterone acetate. Semin. Oncol. 25, 45–52 (1998).
Shedlofsky, S. I., Isreal, B. C., McClain, C. J., Hill, D. B. & Blouin, R. A. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J. Clin. Invest. 94, 2209–2214 (1994).
Heggie, G. D., Sommadossi, J. P., Cross, D. S., Huster, W. J. & Diasio, R. B. Clinical pharmacokinetics of 5-fluorouracil and its metabolism in plasma. Cancer Res. 47, 2203–2206 (1987).
van Kuilenburg, A. B. et al. Heterozygosity for a point mutation in an invariant splice donor site of dihydropyrimidine dehydrogenase and severe 5-fluorouracil related toxicity. Eur. J. Cancer 33, 2258–2264 (1997).
van Kuilenburg, A. B. et al. Lethal outcome of a patient with complete dihydropyrimidine dehydrogenase (DPD) deficiency after administration of 5-fluororuracil: frequency of the common IVS14+1G>A mutation causing DPD deficiency. Clin. Cancer Res. 7, 2832–2839 (2001).
Wei, X. M. Molecular basis of the human dihydropyrimidine dehydrogenase deficiency and 5-fluorouracil toxicity. J. Clin. Invest. 98, 610–615 (1996).
Johnson, M. R. et al. Life-threatening toxicity in a dihydropyrimidine dehydrogenase-deficient patient after treatment with topical 5-fluorouracil. Clin. Cancer Res. 5, 2206–2011 (1999).
Milano, G. et al. Dihydropyrimidine dehydrogenase deficiency and fluorouracil-related toxicity. Br. J. Cancer 79, 627–630 (1999).
Milano, G. & Etienne, M. C. Potential importance of dihydropyrimidine dehydrogenase (DPD) in cancer chemotherapy. Pharmacogenetics 4, 301–306 (1994).
Wells, P. G. et al. Glucuronidation and the UDP-glucuronosyltransferases in health and disease. Drug Metab. Dispos. 32, 281–290 (2004).
Beutler, E., Gelbart, T. & Demina, A. Racial variability in the UDP-glucuronosyltransferase 1 (UGT1A1) promoter: A balanced polymorphism for regulation of bilirubin metabolism? Proc. Natl Acad. Sci. USA 95, 8170–8174 (1998).
Innocenti, F. et al. Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk for severe neutropenia of irinotecan. J. Clin. Oncol. 22, 1382–1388 (2004). A study in humans demonstrating that homozygotes for the UGT1A1 variant are at higher risk of severe neutropaenia and have higher SN-38 AUCs than those who are heterozygous or homozygous for the common allele.
Iida, A. et al. Catalog of 605 single-nucleotide polymorphisms (SNPs) among 13 genes encoding human ATP-binding cassette transporters: ABCA4, ABCA7, ABCA8, ABCD1, ABCD3, ABCD4, ABCE1, ABCF1, ABCG1, ABCG2, ABCG4, ABCG5, and ABCG8. J. Hum. Genet. 47, 285–310 (2002).
Imai, Y. et al. C421A polymorphism in the human breast cancer resistance protein gene is associated with low expression of Q141K protein and low-level drug resistance. Mol. Cancer Ther. 1, 611–616 (2002).
Ito, K., Olsen, S. L., Qiu, W., Deeley, R. G. & Cole, S. P. Mutation of a single conserved tryptophan in multidrug resistance protein 1 (MRP1/ABCC1) results in loss of drug resistance and selective loss of organic anion transport. J. Biol. Chem. 276, 15616–15624 (2001).
Saito, S. et al. Identification of 779 genetic variations in eight genes encoding members of the ATP-binding cassette, subfamily C (ABCC/MRP/CFTR). J. Hum. Genet. 47, 147–171 (2002).
Saito, S. et al. Three hundred twenty-six genetic variations in genes encoding nine members of the ATP-binding cassette, subfamily B (ABCB/MDR/TAP), in the Japanese population. J. Hum. Genet. 47, 38–50 (2002).
Chu, X. Y. et al. Multispecific organic anion transporter is responsible for the biliary excretion of the camptothecin derivative irinotecan and its metabolites in rats. J. Pharmacol. Exp. Ther. 281, 304–314 (1997).
Nakatomi, K. et al. Transport of 7-ethyl-10-hydroxycamptothecin (SN-38) by breast cancer resistance protein ABCG2 in human lung cancer cells. Biochem. Biophys. Res. Commun. 288, 827–832 (2001).
Branch, R. A., Herbert, C. M. & Read, A. E. Determinants of serum antipyrine half-lives in patients with liver disease. Gut 14, 569–573 (1973).
Sotaniemi, E. A., Pelkonen, R. O., Mokka, R. E., Huttunen, R. & Viljakainen, E. Impairment of drug metabolism in patients with liver cancer. Eur. J. Clin. Invest. 7, 269–274 (1977).
Fanucchi, M. P. et al. Phase I and clinical pharmacology study of trimetrexate administered weekly for three weeks. Cancer Res. 47, 3303–3308 (1987).
Ratain, M. J., Vogelzang, N. J. & Sinkule, J. A. Interindividual and intraindividual variability in vinblastine pharmacokinetics. Clin. Pharmacol. Ther. 41, 61–67 (1987).
Balis, F. Pharmacokinetic drug interactions of commonly used anticancer drugs. Clin. Pharmacokinet. 11, 223–235 (1986).
Lippens, R. J. Methotrexate. I. Pharmacology and pharmacokinetics. Am. J. Pediatr. Hematol. Oncol. 6, 379–395 (1984).
Yancik, R. Cancer burden in the aged: an epidemiologic and demographic overview. Cancer 80, 1273–1283 (1997).
Jin, Y. et al. CYP2D6 genotype, antidepressant use, and tamoxifen metabolism during adjuvant breast cancer treatment. J. Natl Cancer Inst. 97, 30–39 (2005).
McCune, J. S., Hatfield, A. J., Blackburn, A. A. & Leith, P. O. Potential of chemotherapy-herb interactions in adult cancer patients. Support. Care. Cancer 12, 454–462 (2004).
Lehmann, J. et al. The human orphan nuclear receptor PXR is activated by compounds that regulate CYP3A4 gene expression and cause drug interactions. J. Clin. Investig. 102, 1016–1023 (1998).
Burk, O. et al. The induction of cytochrome P450 3A5 (CYP3A5) in the human liver and intestine is mediated by the xenobiotic sensors pregnane X receptor (PXR) and constitutively activated receptor (CAR). J. Biol. Chem. 279, 38379–38385 (2004).
Moore, L. B. et al. St. John's wort induces hepatic drug metabolism through activation of the pregnane X receptor. Proc. Natl Acad. Sci. USA 97, 7500–7502 (2000).
Gardner-Stephen, D. et al. Human PXR variants and their differential effects on the regulation of human UDP-glucuronosyltransferase gene expression. Drug Metab. Dispos. 32, 340–347 (2004).
Mathijssen, R. H. J., Verweij, J., de Bruijn P., Loos, W. J. & Sparreboom, A. Effects of St. John's wort on irinotecan metabolism. J. Natl Cancer Inst. 94, 1247–1249 (2002). The results of this study on humans showed that the concomitant use of St John's wort with irinotecan decreases plasma concentrations of SN-38 and reduces the incidence of myelosuppression.
Ho, P. C., Saville, D. J. & Wanwimolruk, S. Inhibition of human CYP3A4 activity by grapefruit flavonoids, furancocoumarins and related compounds. J. Pharm. Pharm. Sci. 4, 217–227 (2001).
Veronese, M. L. et al. Exposure-dependent inhibition of intestinal and hepatic CYP3A4 in vivo by grapefruit juice. J. Clin. Pharmacol. 43, 831–839 (2003).
Mancinelli, L. M. et al. The pharmacokinetics and metabolic disposition of tacrolimus: a comparison across ethnic groups. Clin. Pharmacol. Ther. 69, 24–31 (2001).
Johnson, J. A. Influence of race ethnicity on pharmacokinetics of drugs. J. Pharm. Sci. 86, 1328–1333 (1997).
Schroeder, T. J., Hariharan, S. & First, M. R. Variations in bioavailability of cyclosporine and relationship to clinical outcome in renal transplant subpopulations. Transplant Proc. 27, 837–839 (1995).
Lindholm, A. Welsh, M., Alton, D. & Kahan, B. Demographic factors influencing cyclosporine pharmacokinetic parameters in patients with uremia: racial differences in bioavailability. Transplantation 52, 359–371 (1992).
Lewis, L. D. et al. The pharmacokinetics and pharmacodynamics of docetaxel (DCTX) in Caucasian and African American patients with solid tumors. Proc. Am. Soc. Clin. Oncol. 12, 2043 (2004).
Bruno, R. et al. A population pharmacokinetic model for docetaxel (Taxotere): model building and validation. J. Pharmacokinet. Biopharm. 24, 153–172 (1996).
Smorenburg, C. H. et al. Randomized cross-over evaluation of body-surface area-based dosing versus flat-fixed dosing of paclitaxel. J. Clin. Oncol. 21, 197–202 (2003).
Alberts, D. S. & Dorr, R. T. New perspectives on an old friend: optimizing carboplatin for the treatment of solid tumors. Oncologist 3, 15–34 (1998).
Egorin, M. J. et al. Prospective validation of a pharmacologically based dosing scheme for the cis-diamminedichloroplatinum(II) analogue diamminecyclobutanedicarboxylato-platinum. Cancer Res. 45, 6502–6506 (1985).
Calvert, A. H. et al. Carbopolatin dosage: prospective evaluation of a simple formula based on renal function. J. Clin. Oncol. 7, 1748–1756 (1989). The results of this prospective analysis of a pharmacokinetic-based model for carboplatin dosing show that the model accurately predicts carboplatin AUC.
Dix, S. P. et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 17, 225–230 (1996).
Grochow, L. B. et al. Pharmacokinetics of busulfan: correlations with veno-occlusive disease in patients undergoing bone marrow transplantation. Cancer Chemother. Pharmacol. 25, 55–61 (1989).
Slattery, J. T. et al. Graft-rejection and toxicity following bone marrow transplantation in relation to busulfan pharmacokinetics. Bone Marrow Transplant. 16, 31–42 (1995).
Chattergoon, D. S. et al. An improved limited sampling method for individualised busulphan dosing in bone marrow transplantation in children. Bone Marrow Transplant. 20, 347–354 (1997).
Hassan, M. et al. Busulphan kinetics and limited sampling model in children with leukemia and inherited disorders. Bone Marrow Transplant. 18, 843–850 (1996).
Bleyzac, N. et al. Improved clinical outcome of paediatric bone marrow recipients using a test dose and Bayesian pharmacokinetic individualization of busulfan dosage regimens. Bone Marrow Transplant. 28, 743–751 (2001). In this study on humans, busulfan dosing was based on individual pharmacokinetics that were determined after a test dose, showing that this strategy accurately predicts the AUC of the definitive dose and reduced treatment-related toxicity.
Yamamoto, N. et al. Correlation between docetaxel clearance and estimated cytochrome P450 activity by urinary metabolite of exogenous cortisol. J. Clin. Oncol. 18, 2301–2308 (2000).
Yamamoto, N. et al. Randomized pharmacokinetic and pharmacodynamic study of docetaxel: dosing based on body-surface area compared with individualized dosing based on cytochrome P450 activity estimated using a urinary metabolite of exogenous cortisol. J. Clin. Oncol. 23, 1061–1069 (2005).
Zhang, L., Price, R., Aweeka, F., Bellibas, S. E. & Sheiner, L. B. Making the most of sparse clinical data by using a predictive-model-based analysis, illustrated with a stavudine pharmacokinetic study. Eur. J. Pharm. Sci. 12, 377–385 (2001).
Rousseau, A., Marquet, P., Debord, J., Sabot, C. & Lachatre, G. Adaptive control methods for the dose individualization of anticancer agents. Clin. Pharmacokinet. 38, 315–353 (2000).
Sheiner, L. B. & Steimer, J. L. Pharmacokinetic/pharmacodynamic modeling in drug development. Annu. Rev. Pharmacol. Toxicol. 40, 67–95 (2000).
Jelliffe, R. W. et al. Model-based, goal oriented, individualised drug therapy: linkage of population modeling, new 'multiple model' dosage design, Bayesian feedback and individualised target goals. Clin. Pharmacokinet. 34, 57–77 (1998).
FDA label information [online] < www.fda.gov/cder/foi/label/1999/50778lbl.pdf> (2003).
Ralph, L. D., Thomson, A. H., Dobbs, N. A. & Twelves, C. A population model of epirubicin pharmacokinetics and application to dosage guidelines. Cancer Chemother. Pharmacol. 52, 34–40 (2003).
Jakobsen, P. et al. A randomized study of epirubicin at four different dose levels in advanced breast cancer. Feasibility of myelotoxicity prediction through single blood-sample measurement. Cancer. Chemother. Pharmacol. 28, 465–469 (1991).
Liebmann, J. E. et al. Cytotoxic studies of paclitaxel (Taxol) in human tumour cell lines. Br. J. Cancer 68, 1104–1109 (1993).
Lopes, N. M., Adams, E. G., Pitts, T. W. & Bhuyan, B. K. Cell kill kinetics and cell cycle effects of Taxol on human and hamster ovarian cell lines. Cancer Chemother. Pharmacol. 32, 235–242 (1993).
Raymond, E. et al. Effects of prolonged versus short-term exposure paclitaxel (Taxol) on human tumor colony-forming units. Anticancer Drugs 8, 379–385 (1997).
Meerum Terwogt, J. M. et al. Co-administration of cyclosporin A enables oral therapy with paclitaxel. Clin. Cancer Res. 5, 3379–3384 (1999).
Malingré, M. M. et al. Phase I and pharmacokinetic study of oral paclitaxel. J. Clin. Oncol. 18, 2468–2475 (2000).
Kruijtzer, C. M. F. et al. Phase II and pharmacologic study of weekly oral paclitaxel plus cyclosporine in patients with advanced non-small-cell lung cancer. J. Clin. Oncol. 20, 4508–4516 (2002).
Kruijtzer, C. M. F. et al. Weekly oral paclitaxel as first-line treatment in patients with advanced gastric cancer. Ann. Oncol. 14, 197–204 (2003).
Ten Bokkel Huinink, W. W. et al. Single-agent gemcitabine: an active and better tolerated alternative to standard cisplatin-based therapy in locally advanced or metastatic non-small cell lung cancer. Lung Cancer 26, 85–94 (1999).
Socinski, M. A. Single-agent paclitaxel in the treatment of advanced non-small cell lung cancer. Oncologist 4, 408–416 (1994).
Ranson, M. et al. Randomized trial of paclitaxel plus supportive care versus supportive care for patients with advanced non-small cell lung cancer. J. Natl Cancer Inst. 92, 1074–1080 (2000).
Cullinan, S. A. et al. Controlled evaluation of three drug combination regimens versus fluorouracil alone for the therapy of advanced gastric cancer. J. Clin. Oncol. 12, 412–416 (1994).
Wils, J. A. et al. Sequential high-dose methotrexate and fluorouracil combined with doxorubicin. A step ahead in the treatment of advanced gastric cancer: a trial of the European Organization for Research and Treatment of Cancer Gastrointestinal Tract Cooperative Group. J. Clin. Oncol. 9, 827–831 (1991).
Webb, A. et al. Randomized trial comparing epirubicin, cisplatin, and fluorouracil versus fluorouracil, doxorubicin and methotrexate in advanced esophagogastric cancer. J. Clin. Oncol. 15, 261–267 (1997).
Gupta, E., Safa, A. R., Wang, X. & Ratain, M. J. Pharmacokinetic modulation of irinotecan and metabolites by cyclosporine A. Cancer Res. 56, 1309–1314 (1996).
Chester, J. D. et al. Phase I and pharmacokinetic study of intravenous irinotecan plus oral ciclosporin in patients with fluorouracil-refractory metastatic colon cancer. J. Clin. Oncol. 21, 1125–1132 (2003). The results of this study in humans show that the modulation of irinotecan by cyclosporine increases the AUC of irinotecan and its metabolites, and decreases irinotecan clearance.
Sanathanan, L. P. & Peck, C. C. The randomized concentration-controlled trial: an evaluation of its sample-size efficiency. Control. Clin. Trials 12, 780–794 (1991). This report outlines the design and advantages of the randomized concentration-controlled trial for drugs with narrow therapeutic windows.
Christensen, J., Andreasen, F., Poulsen, J. H. & Dam, M. Randomized, concentration-controlled trial of topiramate in refractory focal epilepsy. Neurology 61, 1210–1218 (2003).
Ratain, M. J. & Relling, M. V. Gazing into a crystal ball-cancer therapy in the post-genomic era. Nature Med. 7, 283–285 (2001).
Benet, L. Z. & Galeazzi, R. L. Noncompartmental determination of the steady-state volume of distribution. J. Pharm. Sci. 68, 1071–1074 (1979).
DiStefano, J. J. III. Noncompartmental vs. compartmental: some basis for choice. Am. J. Physiol. 243, R1–R6 (1982).
Gillespie, W. R. Noncompartmental versus compartmental modeling in clinical pharmacokinetics. Clin. Pharmacokinet. 20, 253–262 (1991).
Metzler, C. M. Usefulness of the two-compartment open model in pharmacokinetics. J. Am. Stat. Assoc. 66, 49–54 (1971).
Gibaldi, M. & Perrier, D. in Pharmacokinetics (ed. Swarbrick, J.) 199–219 (Marcel Dekker, New York, 1982).
Iber, F. L., Murphy, P. A. & Connor, E. S. Age-related changes in the gastrointestinal system: effects on drug therapy. Drugs Aging 5, 34–48 (1994).
Corcoran, M. E. Polypharmacy in the older patient with cancer. Cancer Control 4, 419–428 (1997).
Skirvin, J. A. & Lichtman, S. M. Pharmacokinetic considerations of oral chemotherapy in elderly patients with cancer. Drugs Aging 19, 25–42 (2002).
Johnson, S. L., Mayersohn, M. & Conrad, K. A. Gastrointestinal absorption as a function of age: xylose absorption in healthy adults. Clin. Pharmacol. Ther. 38, 331–335 (1985).
Baker, S. D. & Grochow, L. B. Pharmacology of cancer chemotherapy in the older person. Clin. Geriatr. Med. 13, 169–183 (1997).
Egorin, M. J. Cancer pharmacology in the elderly. Semin. Oncol. 20, 43–49 (1993).
Pierelli, L. et al. Erythropoietin addition to granulocyte colony-stimulating factor abrogates life-threatening neutropenia and increases peripheral-blood progenitor-cell mobilization after epirubicin, paclitaxel, and cisplatin combination chemotherapy: results of a randomized comparison. J. Clin. Oncol. 17, 1288–1295 (1999).
Schrijvers, D., Highley, M., De Bruyn E., Van Oosterom, A. T. & Vermorken, J. B. Role of red blood cells in pharmacokinetics of chemotherapeutic agents. Anticancer Drugs 10, 147–153 (1999).
Extermann, M. et al. Predictors of tolerance to chemotherapy in older cancer patients: a prospective pilot study. Eur. J. Cancer 38, 1466–1473 (2002).
Sotaniemi, E. A., Arranto, A. J., Pelkonen, O. & Pasanen M. Age and cytochrome P450-linked drug metabolism in humans: an analysis of 226 subjects with equal histopathologic conditions. Clin. Pharmacol. Ther. 61, 332–339 (1997).
Vestal, R. E. Aging and pharmacology. Cancer 80, 1302–1310 (1997).
Brenner, B. M., Meyer, G. W. & Hostetter, T. H. Dietary protein intake and the progressive nature of kidney disease: the role of hemodynamically mediated glomerular injury in the pathogenesis of progressive glomerular sclerosis in aging, renal ablation, and intrinsic renal disease. N. Engl. J. Med. 307, 652–659 (1982).
Anderson, S. & Brenner, B. M. Effects of aging on the renal glomerulus. Am. J. Med. 80, 435–442 (1986).
Lichtman, S. M. & Villani, G. Chemotherapy in the elderly: pharmacologic considerations. Cancer Control 7, 548–556 (2000).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- ABSORPTION
-
The process by which a drug leaves its site of administration and enters systemic circulation. Not relevant to intravenously administered agents.
- DISTRIBUTION
-
The process by which a drug distributes into interstitial and intracellular fluids.
- METABOLISM
-
The process by which a drug undergoes biotransformation to a usually inactive (but sometimes active) metabolite.
- EXCRETION
-
The process by which a drug and/or its metabolites are removed from the body.
- THERAPEUTIC INDEX
-
Conceptual description of the ratio of the toxic dose to the therapeutic dose of a drug, describing the dose range over which a drug is therapeutic but not unacceptably toxic.
- COEFFICIENT OF VARIATION
-
The ratio of the standard deviation to the mean, multiplied by 100%. A high coefficient of variation means high interindividual variability.
- BIOAVAILABILITY
-
The fraction of an administered dose that reaches systemic circulation.
- FIRST-PASS EFFECT
-
The decrease in bioavailability of an oral drug owing to enteric metabolism, hepatic metabolism and excretion before the drug reaches the systemic circulation.
- AREA UNDER THE CURVE (AUC).
-
The area under the curve in a graph of plasma concentration against time — a measure of drug exposure.
- POLYMORPHISM
-
The presence of two or more alleles with a frequency of at least 1% in the general population at the same gene locus.
- CYTOCHROME P450 ENZYMES
-
A family of haeme-containing intracellular oxidizing enzymes that are responsible for the first phase of metabolism of many drugs.
- PRODRUGS
-
Pharmacologically inactive derivatives of active drugs that must undergo metabolic conversion to the active agent.
- PHARMACOGENETICS
-
The study of genetically determined variations in drug response.
- PLEURAL EFFUSION
-
An abnormal collection of fluid between the thin layers of tissue (pleura) lining the lung and wall of the chest cavity.
- AMPHIPATHIC
-
Containing both hydrophilic and hydrophobic domains.
- GLOMERULAR FILTRATION RATE
-
A measure of renal function that equals the quantity of glomerular filtrate formed per unit of time in all nephrons of both kidneys.
- VENO-OCCLUSIVE DISEASE
-
A complication of bone-marrow transplantation in which there is obstruction of the terminal venules and/or sinusoids in the liver. Clinical features include hyperbilirubinaemia, painful hepatomegaly and fluid retention.
- BILIRUBIN
-
A breakdown product of haeme that circulates in the plasma, is taken up by the liver and conjugated so that it is water soluble, and is then excreted in the bile. It is routinely measured as a blood test to assess liver function.
Rights and permissions
About this article
Cite this article
Undevia, S., Gomez-Abuin, G. & Ratain, M. Pharmacokinetic variability of anticancer agents. Nat Rev Cancer 5, 447–458 (2005). https://doi.org/10.1038/nrc1629
Issue Date:
DOI: https://doi.org/10.1038/nrc1629
This article is cited by
-
Combination effects of amino acid transporter LAT1 inhibitor nanvuranlat and cytotoxic anticancer drug gemcitabine on pancreatic and biliary tract cancer cells
Cancer Cell International (2023)
-
De novo identification of maximally deregulated subnetworks based on multi-omics data with DeRegNet
BMC Bioinformatics (2022)
-
Association between gene polymorphism and adverse effects in cancer patients receiving docetaxel treatment: a meta-analysis
Cancer Chemotherapy and Pharmacology (2022)
-
The Value of Pharmacogenetics to Reduce Drug-Related Toxicity in Cancer Patients
Molecular Diagnosis & Therapy (2022)
-
A phase 1, open-label, drug–drug interaction study of rucaparib with rosuvastatin and oral contraceptives in patients with advanced solid tumors
Cancer Chemotherapy and Pharmacology (2021)