Key Points
-
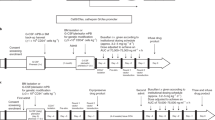
Gene therapy for blood-cell diseases can be performed with retroviral vectors that insert into the genome of haematopoietic stem cells.
-
A recent trial of gene therapy for infants with X-linked severe combined immune deficiency (XSCID) successfully restored the immune systems of most subjects.
-
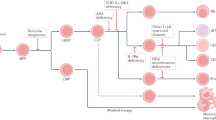
Two subjects developed T-cell leukaemia more than 2 years after gene therapy commenced. This cancer seems to be caused by retroviral-vector activation of a cellular oncogene at the site of integration, a process known as 'insertional oncogenesis'.
-
The complication of leukaemia has not occurred in any other clinical trial, nor in any large animal model that used retroviral vectors to modify haematopoietic stem cells. Leukaemia has been linked to vector integration in only one mouse study using this approach.
-
Multiple factors could have contributed to the development of leukaemia in the patients involved in this trial. These include the high level of engraftment and expansion of the genetically modified cells, unique properties of the haematopoietic stem and progenitor cells in bone marrow of X-linked SCID patients, the immune deficiency of the X-linked SCID patients and/or the transferred gene itself.
-
Further use of current gene-transfer methods for the treatment of SCID poses an ethical dilemma in the consideration of the complex benefits and risks.
-
It might be possible to develop retroviral vectors or other gene-therapy methods that are less likely to lead to insertional oncogenesis and still retain the therapeutic benefits. The use of tissue-specific, regulated transcription units should, in principle, diminish the risk of proto-oncogene transactivation.
Abstract
Recombinant viral vectors have allowed gene transfer to be developed as a promising approach to the treatment of genetic diseases. Recently, gene therapy of children with X-linked severe combined immune deficiency resulted in impressive levels of immune reconstitution — a triumph that was later overshadowed by the development of leukaemia in two patients. What were the causes of this cancer, and how can the therapeutic benefits of gene therapy be achieved while minimizing risk to the patient?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Kohn, D. B. Gene therapy for genetic and haematological disorders and immunodeficiency. J. Int. Med. 249, 379–390 (2001).
Vijaya, S. Acceptor sites for retroviral integrations map near DNase I-hypersensitive sites in chromatin. J. Virol. 60, 683–692 (1986).
Rohdewohld, H. et al. Retrovirus integration and chromatin structure: Moloney murine leukemia proviral integration sites map near DNase I-hypersensitive sites. J. Virol. 61, 336–343 (1987).
Schroder, A. R. et al. HIV-1 integration in the human genome favors active genes and local hotspots. Cell 110, 521–529 (2002). A paper that catalogued more than 500 independent genomic integration sites of HIV in human cells. A modest preference was seen for integration into gene-rich regions that were actively transcribed.
Nakai, H. et al. AAV serotype 2 vectors preferentially integrate into active genes in mice. Nature Genet. 1 Jun 2003 (doi:10.1038/ng1179).
Kotin, R. M. et al. Site specific integration by adeno-associated virus. Proc. Natl Acad. Sci. USA. 87, 2211–2215 (1990).
Strayer, D. S. et al. Durability of transgene expression and vector integration: recombinant SV40-derived gene therapy vectors. Mol. Ther. (in the press).
Miller, A. D. in Retroviruses (eds Coffin, F. M., Hughes, S. H. & Varmus, H. E.) 437–474 (Cold Spring Harbor Laboratory Press, New York, 1997).
Baum, C., Ostertag, W., Stocking, C. & von Laber, D. in Gene Therapy of Cancer (eds Lattime, E. C. & Gerson, S. L.) 3–30 (Academic Press, Missouri, USA, 2002).
Heim, D. A. & Dunbar, C. E. Hematopoietic stem cell gene therapy: towards clinically significant gene transfer efficiency. Immunol. Rev. 178, 29–38 (2000).
Halene, S. & Kohn, D. B. Gene therapy using hematopoietic stem cells: Sisyphus approaches the crest. Hum. Gene Ther. 11, 1259–1267 (2000).
Sadelain, M. et al. Issues in the manufacture and transplantation of genetically modified hematopoietic stem cells. Curr. Opin. Hematol. 7, 364–377 (2000).
Cavazzana-Calvo, M. et al. Gene therapy of human severe combined immunodeficiency (SCID)-X1 disease. Science 288, 669–672 (2000). The first report on the effects of gene therapy in two patients with X-linked SCID subjects. This paper marks the milestone of the first 'cure' of a disease by gene therapy.
Hacein-Bey-Abina, S. et al. Sustained correction of X-linked severe combined immunodeficiency by ex vivo gene therapy. N. Engl. J. Med. 346, 1185–1193 (2002). A second report with longer follow-up of the first two subjects reported in reference 7, with three additional subjects reported. Restoration of immunity was seen in four of five subjects. Two of these subjects subsequently developed T-cell leukaemia.
Weinberg, K. I. & Kohn, D. B. Gene therapy for congenital lymphoid immunodeficiency diseases. Semin. Hematol. 35, 354–366 (1998).
Antoine, C. S. et al. Long-term survival and transplantation of haemopoietic stem cells for immunodeficiencies: report of the European experience 1968–99. Lancet 361, 553–560 (2003).
Aiuti, A. et al. Correction of ADA-SCID by stem cell gene therapy combined with nonmyeloablative conditioning. Science 296, 2410–2413 (2002). The first report of immune restoration in two patients with ADA-deficient SCID. Unlike all previous gene-therapy studies for this form of SCID, these subjects were not treated with PEG-ADA enzyme-replacement therapy, which is believed to reduce the selective advantage of the gene-corrected cells. Additionally, these subjects received bone-marrow cytoreduction with a moderate dosage of the chemotherapeutic agent busulphan, which could promote engraftment of the gentically modified cells.
Marshall, E. Gene therapy. Second child in French trial is found to have leukemia. Science 299, 320 (2003).
Wu, X. & Pandolfi, P. P. Mouse models for multistep tumorigenesis. Trends Cell Biol. 11, S2–S9 (2001).
Rosenberg, N. et al. in Retroviruses (eds Coffin, A. M., Hughes, S. H. & Varmus, H. E.) 475–585 (Cold Spring Harbor Laboratory Press, New York, 1997).
Suzuki, T. et al. New genes involved in cancer identified by retroviral tagging. Nature Genet. 32, 166–174 (2002). In this study (and also references 22 and 23), researchers sequenced retroviral insertional sites in the mouse genome to examine cellular oncogenes that are involved in transformation. Multiple genes in specific signalling pathways were shown to promote transformation.
Mikkers, H., J. et al. High-throughput retroviral tagging to identify components of specific signaling pathways in cancer. Nature Genet. 32, 153–159 (2002).
Joosten, M. et al. Large-scale identification of novel potential disease loci in mouse leukemia applying an improved strategy for cloning common virus integration sites. Oncogene 21, 7247–7255 (2002).
Morishita, K. et al. Retroviral activation of a novel gene encoding a zinc finger protein in IL-3-dependent myeloid leukemia cell line. Cell 54, 831–840 (1988).
Kohn, D. B. et al. Report of the ad hoc sub-committee of the American Society of Gene Therapy on clinical trials conducted using retroviral-mediated gene transfer to hematopoietic stem cells. Mol. Ther. (in the press).
Li, Z. et al. Murine leukemia induced by retroviral gene marking. Science 296, 497 (2002). The first reported observation of insertional oncogenesis by a replication-incompetent retroviral vector. The retroviral vector was inserted near the Evi1 locus — a gene that is associated with acute myeloid leukaemia.
Nucifora, G. in Transcription Factors: Normal and Malignant Development of Blood Cells (eds Ravid, K. & Licht, J. D.) 393–404 (Wiley-Liss, New Jersey, USA 2001).
Osada, H. et al. Association of erythroid transcription factors: complexes involving the LIM protein RBTN2 and the zinc-finger protein GATA1. Proc. Natl Acad. Sci. USA 92, 9585–9589 (1995).
Rabbitts, T. H. et al. The effect of chromosomal translocations in acute leukemias: the LMO2 paradigm in transcription and development. Cancer Res. 59 (7 Suppl), 1794s–1798s (1999).
Royer-Pokora, B., Loos, U. & Ludwig, W. D. TTG-2, a new gene encoding a cysteine-rich protein with the LIM motif, is overexpressed in acute T-cell leukaemia with the t(11;14)(p13;q11). Oncogene 6, 1887–1893 (1991).
Larson, R. C. et al. The oncogenic LIM protein Rbtn2 causes thymic developmental aberrations that precede malignancy in transgenic mice. Oncogene 11, 853–862 (1995).
Neale, G. A, Rehg, J. E. & Goorha, R. M. Ectopic expression of rhombotin-2 causes selective expansion of CD4−CD8− lymphocytes in the thymus and T-cell tumors in transgeneic mice. Blood 86, 3060–3071 (1995).
Rabbits, T. H. Chromosomal translocation master genes, mouse models and experimental therapeutics. Oncogene 20, 5763–5777 (2001).
Ferrando, A. A. et al. Gene expression signatures define novel oncogenic pathways in T cell acute lymphoblastic leukemia. Cancer Cell 1, 75–87 (2002).
Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. Defective IL7R expression in T(−)B(+)NK(+) severe combined immunodeficiency. Nature Genet. 20, 394–397 (1998).
Schluns, K. S., Kieper, W. C., Jameson, S. C. & Lefrancois, L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nature Immunol. 1, 426–432 (2000).
Schluns, K. S. & Lefrancois, L. Cytokine control of memory T-cell development and survival. Nature Rev. Immunol. 3, 269–279 (2003).
Valenzona, H. O. Prelymphomatous B cell hyperplasia in the bone marrow of interleukin-7 transgenic mice: precursor B cell dynamics, microenvironmental organization and osteolysis. Exp. Hematol. 24, 1521–1529 (1996).
Fehniger, T. A. et al. Fatal leukemia in interleukin-15 transgenic mice. Blood Cells Mol. Dis. 27, 223–230 (2001).
Renauld, J. C. et al. Thymic lymphomas in interleukin 9 transgenic mice. Oncogene 9, 1327–1332 (1994).
Lange, K. et al. Overexpression of NPM-ALK induces different types of malignant lymphomas in IL-9 transgenic mice. Oncogene 22, 517–527 (2003).
Fehniger, T. A. & Caligiuri, M. A. Interleukin-15: biology and relevance to human disease. Blood 97, 14–32 (2001).
Barata, J. T., Cardoso, A. A., Nadler, L. M. & Boussiotos, V. A. Interleukin-7 promotes survival and cell cycle progression of T cell acute lymphoblastic leukemia cells by down-regulating the cyclin-dependent kinase inhibitor p27(kip1). Blood 98, 1524–1531 (2001).
Bolotin, E. et al. Serum levels of IL-7 in bone marrow transplant recipients: relationship to clinical characteristics and lymphocyte count. Bone Marrow Transplant. 23, 783–788 (1999).
Sadelain, M. & Riviere, I. Sturm und drang over suicidal lymphocytes. Mol. Ther. 5, 655–657 (2002).
Mavilio, F. et al. Peripheral blood lymphocytes as target cells of retroviral vector-mediated gene transfer. Blood 83, 1988–1997 (1994).
Bonini, C. et al. Safety of retroviral gene marking with a truncated NGF receptor. Nature Med. 9, 367–368 (2003).
Dunn, G. P. et al. Cancer immunoediting: from immunosurveillance to tumor escape. Nature Immunol. 3, 991–998 (2002).
Shankaran, V. et al. IFNgamma and lymphocytes prevent primary tumour development and shape tumour immunogenicity. Nature 410, 1107–1111 (2001).
O'Reilly, R. J. et al. Biology and adoptive cell therapy of EBV-associated lymphoproliferative disorders in recipients of marrow allografts. Immunol. Rev. 157, 195–216 (1997).
Okano, M. & Gross, T. G. A review of Epstein–Barr virus infection in patients with immunodeficiency disorders. Am. J. Med. Sci. 319, 392–396 (2000).
Smyth, M. J., Hayakawa, Y., Takeda, K. & Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nature Rev. Cancer 2, 850–861 (2002).
Soudais, C. et al. Stable and functional lymphoid of common cytokine reception chain deficient mice by retroviral-mediated gene transfer. Blood 95, 3071–3077 (2000).
Valk, P. J. A rapid RT-PCR based method to isolate complementary DNA fragments flanking retrovirus integration sites. Nucleic Acids Res. 25, 4419–4421 (1997).
Schmidt, M. et al. Detection and direct genomic sequencing of multiple rate unknown flanking DNA in highly complex samples. Hum. Gene Ther. 12, 743–749 (2001). Description of the LAM-PCR technique that was used by Christoph von Kalle and colleagues to uncover the LMO2 integration sites in the two leukaemia patients.
Donahue, R. E. et al. Helper virus induced t cell lymphoma in nonhuman primates after retroviral mediated gene transfer. J. Exp. Med. 176, 1125–1135 (1992).
Yu, S. F. et al. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc. Natl Acad. Sci. USA 83, 3194–3198 (1986).
Ismail, S. I., Rohll, J. B., Kingsman, S. M., Kingsman, A. J. & Uden, M. Use of intron-disrupted polyadenylation sites to enhance expression and safety of retroviral vectors. J. Virol. 75, 199–204 (2001).
Zaiss, A. K. et al. RNA 3′ readthrough of oncoretrovirus and lentivirus: implications for vector safety and efficacy. J. Virol. 76, 7209–7219 (2002).
Sadelain, M. Globin gene transfer for the treatment of severe hemoglobinopathies: a paradigm for stem cell-based gene therapy. J. Gene Med. 4, 113–121 (2002).
Rivella, S. & Sadelain, M. Therapeutic globin gene delivery using lentiviral vectors. Curr. Opin. Mol. Ther. 4, 505–514 (2002).
May, C. et al. Therapeutic haemoglobin synthesis in beta-thalassaemic mice expressing lentivirus-encoded human beta-globin. Nature 406, 82–86 (2000). Shows that lentiviral vector-mediated erythroid-specific expression of a globin gene can be used to treat haemoglobinopathies. This was an elusive goal for investigators who were trying the same approach using retroviral vectors, due to instability conferred by the necessary globin genomic regulatory sequences.
Baron, U. Tetracycline-controlled transcription in eukaryotes: novel transactivators with graded transactivation potential. Nucleic Acids Res. 25, 2723–2729 (1997).
Gerasimova, T. I. & Corces, V. G. Chromatin insulators and boundaries: effects on transcription and nuclear organization. Annu. Rev. Genet. 35, 193–208 (2001).
West, A. G., Gazner, M. & Felsenfold, G. Insulators: many functions, many mechanisms. Genes Dev. 16, 271–288 (2002).
Rivella, S. et al. The cHS4 insulator increases the probability of retroviral expression at random chromosomal integration sites. J. Virol. 74, 4679–4687 (2000).
Emery, D. W. et al. A chromatin insulator protects retrovirus vectors from chromosomal position effects. Proc. Natl Acad. Sci. USA 97, 9150–9155 (2000).
Cohen, J. L. et al. Suicide gene-mediated modulation of graft-versus-host disease. Leuk. Lymph. 34, 473–480 (1999).
Boeke, J. D. et al. Yeast retrotransposons: finding a nice quiet neighborhood. Cell 93, 1087–1089 (1998).
Bushman, F. Targeting retroviral integration? Mol. Ther. 6, 570–571 (2002).
Buckley, R. H. et al. Human severe combined immunodeficiency: genetic, phenotypic, and functional diversity in one hundred eight infants. J. Pediatr. 130, 378–387 (1997).
Fischer, A. et al. Naturally occurring primary deficiencies of the immune system. Annu. Rev. Immunol. 15, 93–124 (1997).
Leonard, W. J. The molecular basis of X-linked severe combined immunodeficiency: defective cytokine receptor signaling. Annu. Rev. Med. 47, 229–239 (1996).
Noguchi, M. et al. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell 73, 147–157 (1993).
de Saint Basile, G. et al. Close linkage of the locus for X chromosome-linked severe combined immunodeficiency to polymorphic DNA markers in Xq11–q13. Proc. Natl Acad. Sci. USA 84, 7576–7579 (1987).
Puck, J. M., Nussbaum, R. L., Smead, D. L. & Conley, M. E. X-linked severe combined immunodeficiency: localization within the region Xq13.1-q21.1 by linkage and deletion analysis. Am. J. Hum. Genet. 44, 724–730 (1989).
Giri, J. et al. Elevated levels of shed type II IL-1 receptor in sepsis. Potential role for type II receptor in regulation of IL-1 responses. J. Immunol. 153, 5802–5809 (1994).
Kondo, M. et al. Sharing of the interleukin-2 (IL-2) receptor gamma chain between receptors for IL-2 and IL-4. Science 262, 1874–1877 (1993).
Russell, S. M. et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-4 receptor. Science 262, 1880–1883 (1993).
Noguchi, M. et al. Interleukin-2 receptor gamma chain: a functional component of the interleukin-7 receptor. Science 262, 1877–1880 (1993).
Kondo, M. et al. Functional participation of the IL-2 receptor gamma chain in IL-7 receptor complexes. Science 263, 1453–1454 (1994).
Russell, S. M. et al. Interaction of IL-2R beta and gamma c chains with Jak1 and Jak3: implications for XSCID and XCID. Science 266, 1042–1045 (1994).
Kimura, Y. et al. Sharing of the IL-2 receptor gamma chain with the functional IL-9 receptor complex. Int. Immunol. 7, 115–120 (1995).
Asao, H. et al. Cutting edge: the common gamma-chain is an indispensable subunit of the IL-21 receptor complex. J. Immunol. 167, 1–5 (2001).
Leonard, W. J. Cytokines and immunodeficiency diseases. Nature Rev. Immunol. 1, 200–208 (2001).
Kennedy, M. K. et al. Reversible defects in natural killer and memory CD8 T cell lineages in interleukin 15-deficient mice. J. Exp. Med. 191, 771–780 (2000).
Lodolce, J. P. et al. IL-15 receptor maintains lymphoid homeostasis by supporting lymphocyte homing and proliferation. Immunity 9, 669–676 (1998).
Macchi, P. et al. Mutations of Jak-3 gene in patients with autosomal severe combined immune deficiency (SCID). Nature 377, 65–68 (1995).
Russell, S. M. et al. Mutation of Jak3 in a patient with SCID: essential role of Jak3 in lymphoid development. Science 270, 797–800 (1995).
Coffin, J. M. in Fields Virology (eds Fields, B., Knipe, N., Howley, D. M.) 1767–1848 (Lippincott-Raven, USA, 1996).
Bishop, J. M. Molecular themes in oncogenesis. Cell 64, 235–248 (1991).
Haran–Ghera, N. Potential leukemic cells among bone marrow cells of young AKR/J mice. Proc. Natl Acad. Sci. USA, 77, 2923–2926 (1980).
Asjo, B. et al. Influence of genotype and the organ of origin on the subtype of T-cell in Moloney lymphomas induced by transfer of preleukemic cells from athymic and thymus-bearing mice. Cancer Res. 45, 1040–1045 (1985).
Fan, H., Brightman, B. K., Davis, B. R. & Li, Q. X. in Viruses that Affect the Immune System (ed. Fan, H.Y. et al.) 155–174 (American Society for Microbiology, Washington DC, 1991).
Lazo, P. A., Lee, J. S. & Tsichlis, P. N. Long-distance activation of the Myc protooncogene by provirus insertion in Mlvi-1 or Mlvi-4 in rat T-cell lymphomas. Proc. Natl Acad. Sci. USA 87, 170–173 (1990).
Bartholomew, C. & Ihle, J. N. Retroviral insertions 90 kilobases proximal to the Evi-1 myeloid transforming gene activate transcription from the normal promoter. Mol. Cell. Biol. 11, 1820–1828 (1991).
Corcoran, L. M., Adams, J. M., Dunn, A. R. & Cory, S. Murine T lymphomas in which the cellular myc oncogene has been activated by retroviral insertion. Cell 37, 113–122 (1984).
Selten, G., Cuypers, H. T. & Berns, A. Proviral activation of the putative oncogene Pim-1 in MuLV induced T-cell lymphomas. EMBO J. 4, 1793–1798 (1985).
Author information
Authors and Affiliations
Related links
Related links
DATABASES
Cancer.gov
GenBank
LocusLink
OMIM
FURTHER INFORMATION
The American Society of Gene Therapy
The Center for Biologics Evaluation and Research (CBER), Food and Drug Administration (FDA)
Childrens Hospital Los Angeles
Glossary
- EPISOME
-
A DNA element that persists in the nucleus of a cell, independently of the chromosomal DNA. Episomes might be the genomes of some viruses, including herpesvirus and Epstein–Barr virus, or artifical chromosomes.
- CD34+ STEM AND PROGENITOR CELLS
-
CD34 is a glycoprotein that is present on the surface of approximately 1% of human bone-marrow cells. CD34+ cells have properties of haematopoietic stem-cell and progenitor cells, in that they can proliferate and produce blood cells of various lineages. CD34+ cells can be isolated from the bone marrow or peripheral blood using commercially available immunoaffinity devices, be modified by gene transfer with retroviral vectors and be transplanted back into their donor.
- IMMUNOSCOPE ANALYSIS
-
A method to analyse T-cell diversity and specificity in a sample such as peripheral blood. Immunoscope analysis uses a polymerase-chain-reaction-based assay to examine the rearrangement patterns of T-cell-receptor gene families.
- LIM DOMAIN PROTEIN
-
A cysteine- and histidine-rich, zinc-coordinating domain that is composed of two tandemly repeated zinc fingers. LIM domains do not seem to bind DNA but instead seem to mediate protein–protein interactions.
- U3 REGION OF THE RETROVIRAL LONG TERMINAL REPEAT (LTR)
-
LTRs are the DNA sequences of approximately 600–800 base pairs in length that are present at both ends (5′ and 3′) of the retroviral-vector genome (provirus) — even after integration into the host-cell DNA. The U3 region of the LTR contains strong transcriptional enhancers and a promoter that drives expression of genes that are carried by most retroviral vectors. The U3 of the retroviral vector can activate expression of cellular genes that are adjacent to the site of retroviral integration.
- INSULATORS
-
Insulators are DNA sequences that insert between enhancer and promoter regions of DNA, blocking the ability of the enhancer to activate the promoter. Chains of genes along chromosomes can then act as isolated transcriptional units, and adjacent genes can be regulated independently. Insulators can be used to prevent a vector gene from being influenced by flanking genomic DNA sequences, or, conversely, to protect the flanking cellular genes from being influenced by the vector.
Rights and permissions
About this article
Cite this article
Kohn, D., Sadelain, M. & Glorioso, J. Occurrence of leukaemia following gene therapy of X-linked SCID. Nat Rev Cancer 3, 477–488 (2003). https://doi.org/10.1038/nrc1122
Issue Date:
DOI: https://doi.org/10.1038/nrc1122
This article is cited by
-
Long-term outcomes following CAR T cell therapy: what we know so far
Nature Reviews Clinical Oncology (2023)
-
Chimeric antigen receptor T cells therapy in solid tumors
Clinical and Translational Oncology (2023)
-
CRISPR–Cas9 can cause chromothripsis
Nature Genetics (2021)
-
Are chimeric antigen receptor T cells (CAR-T cells) the future in immunotherapy for autoimmune diseases?
Inflammation Research (2021)
-
Episomal minicircles persist in periods of transcriptional inactivity and can be transmitted through somatic cell nuclear transfer into bovine embryos
Molecular Biology Reports (2019)