Key Points
-
Epidermal stem-cell progeny give rise to the differentiated cells of the interfollicular epidermis (IFE), hair follicles and sebaceous glands.
-
Multipotent epidermal stem cells are likely to be the main target cells for the various types of epidermal tumour.
-
Differentiated cells can regulate the clonal expansion of mutant stem-cell clones.
-
Epidermal cancer comprises many different tumour types, including basal-cell carcinoma (BCC), squamous-cell carcinoma (SCC), trichofolliculoma, pilomatricoma and sebaceous adenoma.
-
Factors responsible for the genesis of specific tumour types have been identified. Some, such as RAS and p53, are important for growth, and others, such as β-catenin, are important for determining differentiated characteristics.
Abstract
The outer covering of the skin — the epidermis — is subject to sustained environmental assaults. As a result, many cells acquire potentially oncogenic mutations. Most cells are lost through differentiation, and only long-term epidermal residents, such as stem cells, accumulate the number of genetic hits that are necessary for tumour development. So, what genetic and environmental factors determine whether a mutant stem cell forms a tumour and what type of tumour will develop?
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Watt, F. M. Stem cell fate and patterning in mammalian epidermis. Curr. Opin. Genet. Dev. 11, 410–417 (2001).
Panteleyev, A. A., Jahoda C. A. & Christiano A. M. Hair follicle predetermination. J. Cell Sci. 114, 3419–3431 (2001).
Niemann, C. & Watt, F. M. Designer skin: lineage commitment in postnatal epidermis. Trends Cell Biol. 12, 185–192 (2002).
Taylor, G., Lehrer, M. S., Jensen, P. J., Sun, T. T. & Lavker, R. M. Involvement of follicular stem cells in forming not only the follicle but also the epidermis. Cell 102, 451–461 (2000). Puts the case that the IFE and sebaceous glands are derived from hair-follicle stem cells.
Oshima, H., Rochat, A., Kedzia, C., Kobayashi, K. & Barrandon, Y. Morphogenesis and renewal of hair follicles from adult multipotent stem cells. Cell 104, 233–245 (2001).
Reynolds, A. J. & Jahoda, C. A. Cultured dermal papilla cells induce follicle formation and hair growth by transdifferentiation of an adult epidermis. Development 115, 587–593 (1992).
Ferraris, C., Bernard, B. A. & Dhouailly, D. Adult epidermal keratinocytes are endowed with pilosebaceous forming abilities. Int. J. Dev. Biol. 41, 491–498 (1997).
Ghazizadeh, S. & Taichman, L. B. Multiple classes of stem cells in cutaneous epithelium: a lineage analysis of adult mouse skin. EMBO J. 20, 1215–1222 (2001). Presents evidence for the existence of discrete stem-cell populations in the IFE, sebaceous glands and hair follicles.
Lavker, R. M. & Sun, T. T. Epidermal stem cells: properties, markers, and location. Proc. Natl Acad. Sci. USA 97, 13473–13475 (2000).
Barrandon, Y. & Green, H. Three clonal types of keratinocyte with different capacities for multiplication. Proc. Natl Acad. Sci. USA 84, 2302–2306 (1987).
Jones, P. H. & Watt, F. M. Separation of human epidermal stem cells from transit amplifying cells on the basis of differences in integrin function and expression. Cell 73, 713–724 (1993).
Reya, T., Morrison, S. J., Clarke, M. F. & Weissman, I. L. Stem cells, cancer, and cancer stem cells. Nature 414, 105–111 (2001).
Nowell, P. C. The clonal evolution of tumor cell populations. Science 194, 23–28 (1976).
Hahn, W. C. et al. Creation of human tumour cells with defined genetic elements. Nature 400, 464–468 (1999).
Hahn, W. C. & Weinberg, R. A. Rules for making human tumor cells. N. Engl. J. Med. 347, 1593–1603 (2002).
Brash, D. E. & Pontén, J. Skin precancer. Cancer Surv. 32, 69–113 (1998).
Jonason, A. S. et al. Frequent clones of p53-mutated keratinocytes in normal human skin. Proc. Natl Acad. Sci. USA 93, 14025–14029 (1996).
Ren, Z. P. et al. Benign clonal keratinocyte patches with p53 mutations show no genetic link to synchronous squamous cell precancer or cancer in human skin. Am. J. Pathol. 150, 1791–1803 (1997).
Jensen, U. B., Lowell, S. & Watt, F. M. The spatial relationship between stem cells and their progeny in the basal layer of human epidermis: a new view based on whole-mount labelling and lineage analysis. Development 126, 2409–2418 (1999).
Brash, D. E. Sunlight and the onset of skin cancer. Trends Genet. 13, 410–414 (1997).
Zhang, W., Remenyik, E., Zelterman, D., Brash, D. E. & Wikonkal, N. M. Escaping the stem cell compartment: sustained UVB exposure allows p53-mutant keratinocytes to colonize adjacent epidermal proliferating units without incurring additional mutations. Proc. Natl Acad. Sci. USA 98, 13948–13953 (2001). Examines the role of UV-B irradiation in driving clonal expansion of cells with TP53 mutations.
Perez–Losada, J. & Balmain, A. Stem cell hierarchy in epithelial cancers. Nature Rev. Cancer 3, 432–441 (2003).
Owens, D. M., Wei, S.-J. C. & Smart, R. C. A multihit, multistage model of chemical carcinogenesis. Carcinogenesis 20, 1837–1844 (1999).
Bailleul, B. et al. Skin hyperkeratosis and papilloma formation in transgenic mice expressing a ras oncogene from a suprabasal keratin promoter. Cell 62, 697–708 (1990).
Greenhalgh, D. A. et al. Induction of epidermal hyperplasia, hyperkeratosis, and papillomas in transgenic mice by a targeted v-Ha-ras oncogene. Mol. Carcinog. 7, 99–110 (1993).
Brown, K., Strathdee, D., Bryson, S., Lambie, W. & Balmain, A. The malignant capacity of skin tumours induced by expression of a mutant H-ras transgene depends on the cell type targeted. Curr. Biol. 8, 516–524 (1998).
Pelengaris, S., Littlewood, T., Khan, M., Elia, G. & Evan, G. Reversible activation of c-Myc in skin: induction of a complex neoplastic phenotype by a single oncogenic lesion. Mol. Cell 3, 565–577 (1999).
Arnold, I. & Watt, F. M. c-Myc activation in transgenic mouse epidermis results in mobilization of stem cells and differentiation of their progeny. Curr. Biol. 11, 558–568 (2001).
Gandarillas, A. & Watt, F. M. c-Myc promotes differentiation of human epidermal stem cells. Genes Dev. 11, 2869–2882 (1997).
Waikel, R. L., Kawachi, Y., Waikel, P. A., Wang, X. J. & Roop, D. R. Deregulated expression of c-Myc depletes epidermal stem cells. Nature Genet. 28, 165–168 (2001).
Frye, M., Gardner, C., Li, E. R., Arnold, I. & Watt, F. M. Evidence that Myc activation depletes the epidermal stem cell compartment by modulating adhesive interactions with the local microenvironment. Development 130, 2793–2808 (2003). References 27–31 show the differing roles of c-MYC in epidermal stem cells and differentiated cells.
Evans, R. D. et al. A tumor-associated β1 integrin mutation that abrogates epithelial differentiation control. J. Cell Biol. 160, 589–596 (2003).
Watt, F. M. Role of integrins in regulating epidermal adhesion, growth and differentiation. EMBO J. 21, 3919–3926 (2002).
Barrandon, Y., Morgan, J. R., Mulligan, R. C. & Green, H. Restoration of growth potential in paraclones of human keratinocytes by a viral oncogene. Proc. Natl Acad. Sci. USA 86, 4102–4106 (1989).
Bonnet, D. & Dick, J. E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nature Med. 3, 730–737 (1997).
Traver, D., Akashi, K., Weissman, I. L. & Lagasse, E. Mice defective in two apoptosis pathways in the myeloid lineage develop acute myeloblastic leukemia. Immunity 9, 47–57 (1998).
Owens, D. M. & Watt, F. M. Influence of β1 integrins on epidermal squamous cell carcinoma formation in a transgenic mouse model: α3β1, but not α2β1, suppresses malignant conversion. Cancer Res. 61, 5248–5254 (2001).
Szabowski, A. et al. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell 103, 745–755 (2000).
Hobbs, R. M. & Watt, F. M. Regulation of interleukin-1α expression by integrins and epidermal growth factor receptor in keratinocytes from a mouse model of inflammatory skin disease. J. Biol. Chem. (in the press).
Akhurst, R. J., Fee, F. & Balmain, A. Localized production of TGF-β mRNA in tumour promoter-stimulated mouse epidermis. Nature 331, 363–365 (1988).
Akhurst, R. J. TGF-β antagonists: why suppress a tumor suppressor? J. Clin. Invest. 109, 1533–1536 (2002).
Vasioukhin, V., Bauer, C., Degenstein, L., Wise, B. & Fuchs, E. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 104, 605–617 (2001).
Perez-Moreno, M., Jamora, C. & Fuchs, E. Sticky business. Orchestrating cellular signals at adherens junctions. Cell 112, 535–548 (2003).
Sawey, M. J., Goldschmidt, M. H., Risek, B., Gilula, N. B. & Lo, C. W. Perturbation in connexin 43 and connexin 26 gap-junction expression in mouse skin hyperplasia and neoplasia. Mol. Carcinog. 17, 49–61 (1996).
Yamakage, K., Omori, Y., Zaidan-Dagli, M. L., Cros, M. P. & Yamasaki, H. Induction of skin papillomas, carcinomas, and sarcomas in mice in which the connexin 43 gene is heterologously deleted. J. Invest. Dermatol. 114, 289–294 (2000).
van Steensel, M. A., van Geel, M., Nahuys, M., Smitt, J. H. & Steijlen, P. M. A novel connexin 26 mutation in a patient diagnosed with keratitis-ichthyosis-deafness syndrome. J. Invest. Dermatol. 118, 724–727 (2002).
Lowell, S., Jones, P., Le Roux, I., Dunne, J. & Watt, F. M. Stimulation of human epidermal differentiation by Delta-Notch signalling at the boundaries of stem-cell clusters. Curr. Biol. 10, 491–500 (2000).
Lowell, S. & Watt, F. M. Delta regulates keratinocyte spreading and motility independently of differentiation. Mech. Dev. 107, 133–140 (2001).
Rangarajan, A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001).
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nature Genet. 33, 416–421 (2003).
Lever, W. F. & Schaumburg–Lever, G. Histopathology of the Skin 6th edn (J.B. Lippincott Company, Philadelphia, 1983).
Bogovski, P. in Pathology of Tumours in Laboratory Animals 1–45 (eds Turusov, V. & Mohr, U.) Vol. 2 (IARC, Lyon, 1994).
Dahmane, N., Lee, J., Robins, P., Heller, P. & Ruiz i Altaba, A. Activation of the transcription factor Gli1 and the Sonic hedgehog signalling pathway in skin tumours. Nature 389, 876–881 (1997).
Oro, A. E. et al. Basal cell carcinomas in mice overexpressing sonic hedgehog. Science 276, 817–821 (1997). This study was the first to show the tumorigenic consequences of altered SHH signalling in an experimental mouse model.
Dajee, M. et al. NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421, 639–643 (2003).
Vassar, R., Hutton, M. E. & Fuchs, E. Transgenic overexpression of transforming growth factor alpha bypasses the need for c-Ha-ras mutations in mouse skin tumorigenesis. Mol. Cell. Biol. 12, 4643–4653 (1992).
Dominey, A. M. et al. Targeted overexpression of transforming growth factor alpha in the epidermis of transgenic mice elicits hyperplasia, hyperkeratosis, and spontaneous, squamous papillomas. Cell Growth Differ. 4, 1071–1082 (1993).
Sibilia, M. et al. The EGF receptor provides an essential survival signal for SOS-dependent skin tumor development. Cell 102, 211–220 (2000).
Malliri, A. et al. Mice deficient in the Rac activator Tiam1 are resistant to Ras-induced skin tumours. Nature 417, 867–871 (2002).
Roper, E. et al. P19(ARF)-independent induction of p53 and cell cycle arrest by Raf in murine keratinocytes. EMBO Rep. 2, 145–150 (2001).
Lin, A. W. & Lowe, S. W. Oncogenic ras activates the ARF-p53 pathway to suppress epithelial cell transformation. Proc. Natl Acad. Sci. USA 98, 5025–5030 (2001).
Dajee, M., Tarutani, M., Deng, H., Cai, T. & Khavari, P. A. Epidermal Ras blockade demonstrates spatially localized Ras promotion of proliferation and inhibition of differentiation. Oncogene 21, 1527–1538 (2002).
Tarutani, M., Cai, T., Dajee, M. & Khavari, P. A. Inducible activation of Ras and Raf in adult epidermis. Cancer Res. 63, 319–323 (2003).
Li, J. et al. PTEN, a putative protein tyrosine phosphatase gene mutated in human brain, breast, and prostate cancer. Science 275, 1943–1947 (1997).
Liaw, D. et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nature Genet. 16, 64–67 (1997). References 64 and 65 provide the identification and molecular characterization of a critical tumour-suppressor gene through the discovery of hereditary mutations that are linked to certain human cancers.
Suzuki, A. et al. Keratinocyte-specific Pten deficiency results in epidermal hyperplasia, accelerated hair follicle morphogenesis and tumor formation. Cancer Res. 63, 674–681 (2003).
Oro, A. E. & Scott, M. P. Splitting hairs: dissecting roles of signaling systems in epidermal development. Cell 95, 575–578 (1998).
Callahan, C. A. & Oro, A. E. Monstrous attempts at adnexogenesis: regulating hair follicle progenitors through Sonic hedgehog signaling. Curr. Opin. Genet. Dev. 11, 541–546 (2001).
Mill, P. et al. Sonic hedgehog-dependent activation of Gli2 is essential for embryonic hair follicle development. Genes Dev. 17, 282–294 (2003).
Toftgård, R. Hedgehog signalling in cancer. Cell. Mol. Life Sci. 57, 1720–1731 (2000).
Bonifas, J. M. et al. Activation of expression of hedgehog target genes in basal cell carcinomas. J. Invest. Dermatol. 116, 739–742 (2001).
Nilsson, M. et al. Induction of basal cell carcinomas and trichoepitheliomas in mice overexpressing GLI-1. Proc. Natl Acad. Sci. USA 97, 3438–3443 (2000).
Grachtchouk, M. et al. Basal cell carcinomas in mice overexpressing Gli2 in skin. Nature Genet. 24, 216–217 (2000).
Gailani, M. R. et al. The role of the human homologue of Drosophila patched in sporadic basal cell carcinomas. Nature Genet. 14, 78–81 (1996).
Fan, H., Oro, A. E., Scott, M. P. & Khavari, P. A. Induction of basal cell carcinoma features in transgenic human skin expressing sonic hedgehog. Nature Med. 3, 788–792 (1997).
Xie, J. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90–92 (1998).
Ling, G. et al. PATCHED and p53 gene alterations in sporadic and hereditary basal cell cancer. Oncogene 20, 7770–7778 (2001).
DasGupta, R. & Fuchs, E. Multiple roles for activated LEF/TCF transcription complexes during hair follicle development and differentiation. Development 126, 4557–4568 (1999).
Reddy, S. et al. Characterization of Wnt gene expression in developing and postnatal hair follicles and identification of Wnt5a as a target of Sonic hedgehog in hair follicle morphogenesis. Mech. Dev. 107, 69–82 (2001).
Gat, U., DasGupta, R., Degenstein, L. & Fuchs, E. De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated β-catenin in skin. Cell 95, 605–614 (1998). The first study to show the tumorigenic effects of misregulated β-catenin signalling in skin and to indicate a role for β-catenin in post-natal epidermal lineage commitment.
Jamora, C., DasGupta, R., Kocieniewski, P. & Fuchs E. Links between signal transduction, transcription and adhesion in epithelial bud development. Nature 422, 317–322 (2003).
Huelsken, J., Vogel, R., Erdmann, B., Cotsarelis, G. & Birchmeier, W. β-Catenin controls hair follicle morphogenesis and stem cell differentiation in the skin. Cell 105, 533–545 (2001).
Merrill, B. J., Gat, U., DasGupta, R. & Fuchs, E. Tcf3 and Lef1 regulate lineage differentiation of multipotent stem cells in skin. Genes Dev. 15, 1688–1705 (2001).
Niemann, C., Owens, D. M., Hulsken, J., Birchmeier, W. & Watt, F. M. Expression of ΔNLef1 in mouse epidermis results in differentiation of hair follicles into squamous epidermal cysts and formation of skin tumours. Development 129, 95–109 (2002).
Andl, T., Reddy, S. T., Gaddapara, T. & Millar, S. E. WNT signals are required for the initiation of hair follicle development. Dev. Cell 2, 643–653 (2002).
Chan, E. F., Gat, U., McNiff, J. M. & Fuchs, E. A common human skin tumour is caused by activating mutations in β-catenin. Nature Genet. 21, 410–413 (1999).
Ingham, P. W. & McMahon, A. P. Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 (2001).
Taipale, J. & Beachy, P. A. The Hedgehog and Wnt signaling pathways in cancer. Nature 411, 349–354 (2001).
Mullor, J. L., Dahmane, N., Sun, T. & Ruiz i Altaba, A. Wnt signals are targets and mediators of Gli function. Curr. Biol. 11, 769–773 (2001).
Al-Hajj, M., Wicha, M. S., Benito-Hernandez, A., Morrison, S. J. & Clarke, M. F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl Acad. Sci. USA 100, 3983–3988 (2003).
Bignell, G. R. et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nature Genet. 25, 160–165 (2000). Description of the CYLD gene, which is mutated in apocrine- and eccrine-gland tumours.
Reitmair, A. H. et al. Spontaneous intestinal carcinomas and skin neoplasms in Msh2-deficient mice. Cancer Res. 56, 3842–3849 (1996).
Fong, L. Y. et al. Muir–Torre-like syndrome in Fhit-deficient mice. Proc. Natl Acad. Sci. USA 97, 4742–4747 (2000).
Kruse, R. et al. 'Second hit' in sebaceous tumors from Muir–Torre patients with germline mutations in MSH2: allele loss is not the preferred mode of inactivation. J. Invest. Dermatol. 116, 463–465 (2001).
Johnson, P. J. & Heckler, F. Muir–Torre syndrome. Ann. Plast. Surg. 40, 676–677 (1998).
Nickerson, M. L. et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with Birt–Hogg–Dubé syndrome. Cancer Cell 2, 157–164 (2002). Identification of folliculin, which is mutated in fibrofolliculomas — hair-follicle tumours in which epithelial strands extend from the infundibulum (neck) of the hair follicle into hyperproliferative connective tissue.
Acknowledgements
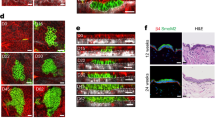
The authors are grateful to Cancer Research UK and a European Union Quality of Life Network grant for financial support. We thank C. Lo Celso, D. Prowse and C. Niemann for providing micrographs for figure 1 and S. Lyle for his advice.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
Glossary
- APOCRINE GLAND
-
A tubular gland of secretory cells that functions primarily to produce scent.
- ECCRINE GLAND
-
A tubular gland of secretory cells that functions primarily in heat regulation through the production of sweat.
- KERATINOCYTE
-
The most abundant cell type in the epidermis. Keratinocytes are epithelial cells and produce hair follicles, sweat and sebaceous glands, and the outer covering of the skin.
- INTEGRINS
-
Heterodimeric transmembrane glycoproteins that are receptors for extracellular-matrix proteins.
- DMBA
-
(7,12-Dimethylbenz[a]anthracene). Bay-region diol-epoxide-type chemical carcinogen. Induces an A→T transversion of codon 61 in Hras.
- TUMOUR PROMOTER
-
Induces epigenetic changes that select for clonal expansion of mutated cells. The phorbol ester type, TPA, is the most widely used tumour promoter in mouse skin carcinogenesis models.
- ADHERENS JUNCTION
-
A cell–cell adhesive junction that contains classical cadherins.
- GAP JUNCTION
-
A cell–cell junction that mediates intercellular communication.
- COWDEN DISEASE
-
Autosomal-dominant disease that features multiple trichilemmomas.
- GORLIN'S SYNDROME
-
A hereditary predisposition to basal-cell carcinomas.
- FAMILIAL CYLINDROMATOSIS
-
Autosomal dominantly inherited syndrome in which tumours have eccrine or apocrine glandular differentiation.
- MUIR–TORRE SYNDROME
-
A condition that results in multiple sebaceous tumours and multiple visceral carcinomas.
- MISMATCH REPAIR
-
A genomic system that detects and repairs incorrectly paired nucleotides that are introduced during DNA replication. Proteins involved include MSH2 and MLH1.
- MICROSATELLITE INSTABILITY
-
Characterized by the accumulation of somatic alterations in the length of simple, repeated nucleotide sequences (called 'microsatellites').
- BIRT–HOGG–DUBE SYNDROME
-
A condition that is characterized by hair-follicle and kidney tumours.
Rights and permissions
About this article
Cite this article
Owens, D., Watt, F. Contribution of stem cells and differentiated cells to epidermal tumours. Nat Rev Cancer 3, 444–451 (2003). https://doi.org/10.1038/nrc1096
Issue Date:
DOI: https://doi.org/10.1038/nrc1096
This article is cited by
-
Advances in cutaneous squamous cell carcinoma
Nature Reviews Cancer (2023)
-
UV filters and high refractive index materials based on carboxymethyl cellulose sodium and CuO@ZnO core/shell nanoparticles
Scientific Reports (2023)
-
Structural and UV-blocking properties of carboxymethyl cellulose sodium/CuO nanocomposite films
Scientific Reports (2023)
-
Cellular Heterogeneity and Plasticity of Skin Epithelial Cells in Wound Healing and Tumorigenesis
Stem Cell Reviews and Reports (2022)
-
NR2F2 controls malignant squamous cell carcinoma state by promoting stemness and invasion and repressing differentiation
Nature Cancer (2021)