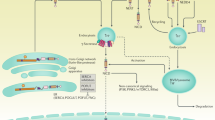
Key Points
-
Notch signalling can be either oncogenic or tumour suppressive depending on the tissue and/or cellular context.
-
Notch signalling is tumour suppressive for various solid tumours, including squamous cell carcinoma in several epithelial tissues, subtypes of brain cancer, liver cancer and small-cell lung cancer.
-
Loss of Notch signalling can result in perturbed regulation of cell fate decisions in stem and progenitor cells, resulting in tumour development.
-
Loss of Notch signalling can also lead to stromal remodelling and the generation of a pro-tumorigenic microenvironment that promotes carcinogenesis.
Abstract
The Notch signalling cascade is an evolutionarily conserved pathway that has a crucial role in regulating development and homeostasis in various tissues. The cellular processes and events that it controls are diverse, and continued investigation over recent decades has revealed how the role of Notch signalling is multifaceted and highly context dependent. Consistent with the far-reaching impact that Notch has on development and homeostasis, aberrant activity of the pathway is also linked to the initiation and progression of several malignancies, and Notch can in fact be either oncogenic or tumour suppressive depending on the tissue and cellular context. The Notch pathway therefore represents an important target for therapeutic agents designed to treat many types of cancer. In this Review, we focus on the latest developments relating specifically to the tumour-suppressor activity of Notch signalling and discuss the potential mechanisms by which Notch can inhibit carcinogenesis in various tissues. Potential therapeutic strategies aimed at restoring or augmenting Notch-mediated tumour suppression will also be highlighted.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Wilson, A. & Radtke, F. Multiple functions of Notch signaling in self-renewing organs and cancer. FEBS Lett. 580, 2860–2868 (2006).
Liu, J., Sato, C., Cerletti, M. & Wagers, A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 92, 367–409 (2010).
Koch, U. & Radtke, F. Notch and cancer: a double-edged sword. Cell. Mol. Life Sci. 64, 2746–2762 (2007).
Radtke, F. & Raj, K. The role of Notch in tumorigenesis: oncogene or tumour suppressor? Nat. Rev. Cancer 3, 756–767 (2003).
Koch, U. & Radtke, F. Notch in T-ALL: new players in a complex disease. Trends Immunol. 32, 434–442 (2011).
Koch, U. & Radtke, F. Mechanisms of T cell development and transformation. Annu. Rev. Cell Dev. Biol. 27, 539–562 (2011).
Teodorczyk, M. & Schmidt, M. H. Notching on cancer's door: Notch signaling in brain tumors. Front. Oncol. 4, 341 (2014).
Reedijk, M. Notch signaling and breast cancer. Adv. Exp. Med. Biol. 727, 241–257 (2012).
McAuliffe, S. M. et al. Targeting Notch, a key pathway for ovarian cancer stem cells, sensitizes tumors to platinum therapy. Proc. Natl Acad. Sci. USA 109, E2939–E2948 (2012).
Galluzzo, P. & Bocchetta, M. Notch signaling in lung cancer. Expert Rev. Anticancer Ther. 11, 533–540 (2011).
Andersson, E. R., Sandberg, R. & Lendahl, U. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612 (2011).
Logeat, F. et al. The Notch1 receptor is cleaved constitutively by a furin-like convertase. Proc. Natl Acad. Sci. USA 95, 8108–8112 (1998).
Gordon, W. R. et al. Structural basis for autoinhibition of Notch. Nat. Struct. Mol. Biol. 14, 295–300 (2007).
Kopan, R. & Ilagan, M. X. The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137, 216–233 (2009).
Dunwoodie, S. L., Henrique, D., Harrison, S. M. & Beddington, R. S. Mouse Dll3: a novel divergent Delta gene which may complement the function of other Delta homologues during early pattern formation in the mouse embryo. Development 124, 3065–3076 (1997).
Ladi, E. et al. The divergent DSL ligand Dll3 does not activate Notch signaling but cell autonomously attenuates signaling induced by other DSL ligands. J. Cell Biol. 170, 983–992 (2005).
Bozkulak, E. C. & Weinmaster, G. Selective use of ADAM10 and ADAM17 in activation of Notch1 signaling. Mol. Cell. Biol. 29, 5679–5695 (2009).
Fortini, M. E. Gamma-secretase-mediated proteolysis in cell-surface-receptor signalling. Nat. Rev. Mol. Cell Biol. 3, 673–684 (2002).
Gordon, W. R. et al. Structure of the Notch1-negative regulatory region: implications for normal activation and pathogenic signaling in T-ALL. Blood 113, 4381–4390 (2009).
Baron, M. Endocytic routes to Notch activation. Semin. Cell Dev. Biol. 23, 437–442 (2012).
Hori, K., Sen, A., Kirchhausen, T. & Artavanis-Tsakonas, S. Regulation of ligand-independent Notch signal through intracellular trafficking. Commun. Integr. Biol. 5, 374–376 (2012).
Palmer, W. H. & Deng, W. M. Ligand-independent mechanisms of Notch activity. Trends Cell Biol. 25, 697–707 (2015).
Bray, S. J. Notch signalling: a simple pathway becomes complex. Nat. Rev. Mol. Cell Biol. 7, 678–679 (2006).
Ishibashi, M. Molecular mechanisms for morphogenesis of the central nervous system in mammals. Anat. Sci. Int. 79, 226–234 (2004).
Holmberg, J. et al. SoxB1 transcription factors and Notch signaling use distinct mechanisms to regulate proneural gene function and neural progenitor differentiation. Development 135, 1843–1851 (2008).
Koch, U., Lehal, R. & Radtke, F. Stem cells living with a Notch. Development 140, 689–704 (2013).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Riccio, O. et al. Loss of intestinal crypt progenitor cells owing to inactivation of both Notch1 and Notch2 is accompanied by derepression of CDK inhibitors p27Kip1 and p57Kip2. EMBO Rep. 9, 377–383 (2008).
Pellegrinet, L. et al. Dll1- and dll4-mediated notch signaling are required for homeostasis of intestinal stem cells. Gastroenterology 140, 1230–1240 (2011).
Blanpain, C. & Fuchs, E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nat. Rev. Mol. Cell Biol. 10, 207–217 (2009).
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157 (2011).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
Pickering, C. R. et al. Mutational landscape of aggressive cutaneous squamous cell carcinoma. Clin. Cancer Res. 20, 6582–6592 (2014).
South, A. P. et al. NOTCH1 mutations occur early during cutaneous squamous cell carcinogenesis. J. Invest. Dermatol. 134, 2630–2638 (2014).
Wang, N. J. et al. Loss-of-function mutations in Notch receptors in cutaneous and lung squamous cell carcinoma. Proc. Natl Acad. Sci. USA 108, 17761–17766 (2011).
Rampias, T. et al. A new tumor suppressor role for the Notch pathway in bladder cancer. Nat. Med. 20, 1199–1205 (2014).
Gao, Y. B. et al. Genetic landscape of esophageal squamous cell carcinoma. Nat. Genet. 46, 1097–1102 (2014).
Song, Y. et al. Identification of genomic alterations in oesophageal squamous cell cancer. Nature 509, 91–95 (2014).
The Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507, 315–322 (2014).
Pickering, C. R. et al. Integrative genomic characterization of oral squamous cell carcinoma identifies frequent somatic drivers. Cancer Discov. 3, 770–781 (2013).
Abel, E. L., Angel, J. M., Kiguchi, K. & DiGiovanni, J. Multi-stage chemical carcinogenesis in mouse skin: fundamentals and applications. Nat. Protoc. 4, 1350–1362 (2009).
Nassar, D., Latil, M., Boeckx, B., Lambrechts, D. & Blanpain, C. Genomic landscape of carcinogen-induced and genetically induced mouse skin squamous cell carcinoma. Nat. Med. 21, 946–954 (2015). This study provided a comprehensive analysis of the genomic aberrations that occur in genetic and chemically induced mouse SCC.
Nicolas, M. et al. Notch1 functions as a tumor suppressor in mouse skin. Nat. Genet. 33, 416–421 (2003). This work provided the first functional in vivo evidence that NOTCH1 functions as a tumour suppressor in non-melanoma skin cancer.
Extance, A. Alzheimer's failure raises questions about disease-modifying strategies. Nat. Rev. Drug Discov. 9, 749–751 (2010).
Maraver, A. et al. NOTCH pathway inactivation promotes bladder cancer progression. J. Clin. Invest. 125, 824–830 (2015). This study presented in vivo functional evidence that Notch signalling functions as a tumour suppressor in the bladder.
Alcolea, M. P. et al. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat. Cell Biol. 16, 615–622 (2014). This work demonstrated how impaired Notch signalling in oesophageal epithelial progenitor cells could result in perturbed differentiation promoting 'field change' in the oesophagus.
Zeng, Q. et al. Crosstalk between tumor and endothelial cells promotes tumor angiogenesis by MAPK activation of Notch signaling. Cancer Cell 8, 13–23 (2005).
Gokulan, R. & Halagowder, D. Expression pattern of Notch intracellular domain (NICD) and Hes-1 in preneoplastic and neoplastic human oral squamous epithelium: their correlation with c-Myc, clinicopathological factors and prognosis in oral cancer. Med. Oncol. 31, 126 (2014).
Yoshida, R. et al. The pathological significance of Notch1 in oral squamous cell carcinoma. Lab. Invest. 93, 1068–1081 (2013).
Zhao, Z. L. et al. NOTCH1 inhibition enhances the efficacy of conventional chemotherapeutic agents by targeting head neck cancer stem cell. Sci. Rep. 6, 24704 (2016).
Hijioka, H. et al. Upregulation of Notch pathway molecules in oral squamous cell carcinoma. Int. J. Oncol. 36, 817–822 (2010).
Yao, J., Duan, L., Fan, M. & Wu, X. Gamma-secretase inhibitors exerts antitumor activity via down-regulation of Notch and nuclear factor kappa B in human tongue carcinoma cells. Oral Dis. 13, 555–563 (2007).
George, J. et al. Comprehensive genomic profiles of small cell lung cancer. Nature 524, 47–53 (2015). This paper identified loss-of-function mutations in Notch family members in SCLC and provided in vivo evidence of a tumour-suppressor role of Notch signalling using a mouse model.
Viatour, P. et al. Notch signaling inhibits hepatocellular carcinoma following inactivation of the RB pathway. J. Exp. Med. 208, 1963–1976 (2011). This study demonstrated that the use of a GSI to inhibit Notch signalling in a mouse model of HCC resulted in impaired tumour development.
Kawaguchi, K. et al. Jagged1 DNA copy number variation is associated with poor outcome in liver cancer. Am. J. Pathol. 186, 2055–2067 (2016).
Ma, L. et al. Overexpression of protein O-fucosyltransferase 1 accelerates hepatocellular carcinoma progression via the Notch signaling pathway. Biochem. Biophys. Res. Commun. 473, 503–510 (2016).
Giachino, C. et al. A tumor suppressor function for Notch signaling in forebrain tumor subtypes. Cancer Cell 28, 730–742 (2015). This study used a mouse model of glioma to functionally demonstrate for the first time in vivo that Notch signalling functions as a tumour suppressor in forebrain tumours.
Alcantara Llaguno, S. et al. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell 15, 45–56 (2009).
Singh, S. K. et al. Identification of human brain tumour initiating cells. Nature 432, 396–401 (2004).
Brat, D. J. et al. Comprehensive, integrative genomic analysis of diffuse lower-grade gliomas. N. Engl. J. Med. 372, 2481–2498 (2015).
Suzuki, H. et al. Mutational landscape and clonal architecture in grade II and III gliomas. Nat. Genet. 47, 458–468 (2015).
Fan, X. et al. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells 28, 5–16 (2010).
Purow, B. W. et al. Expression of Notch-1 and its ligands, Delta-like-1 and Jagged-1, is critical for glioma cell survival and proliferation. Cancer Res. 65, 2353–2363 (2005).
Wang, J. et al. Notch promotes radioresistance of glioma stem cells. Stem Cells 28, 17–28 (2010).
Xu, P. et al. The oncogenic roles of Notch1 in astrocytic gliomas in vitro and in vivo. J. Neurooncol. 97, 41–51 (2010).
Krop, I. et al. Phase I pharmacologic and pharmacodynamic study of the gamma secretase (Notch) inhibitor MK-0752 in adult patients with advanced solid tumors. J. Clin. Oncol. 30, 2307–2313 (2012).
Schonberg, D. L., Lubelski, D., Miller, T. E. & Rich, J. N. Brain tumor stem cells: molecular characteristics and their impact on therapy. Mol. Aspects Med. 39, 82–101 (2014).
Crabtree, J. S., Singleton, C. S. & Miele, L. Notch signaling in neuroendocrine tumors. Front. Oncol. 6, 94 (2016).
Kunnimalaiyaan, M., Vaccaro, A. M., Ndiaye, M. A. & Chen, H. Overexpression of the NOTCH1 intracellular domain inhibits cell proliferation and alters the neuroendocrine phenotype of medullary thyroid cancer cells. J. Biol. Chem. 281, 39819–39830 (2006).
Kunnimalaiyaan, M., Yan, S., Wong, F., Zhang, Y. W. & Chen, H. Hairy enhancer of split-1 (HES-1), a Notch1 effector, inhibits the growth of carcinoid tumor cells. Surgery 138, 1137–1142 (2005).
Nakakura, E. K. et al. Regulation of neuroendocrine differentiation in gastrointestinal carcinoid tumor cells by notch signaling. J. Clin. Endocrinol. Metab. 90, 4350–4356 (2005).
Rekhtman, N. et al. Next-generation sequencing of pulmonary large cell neuroendocrine carcinoma reveals small cell carcinoma-like and non-small cell carcinoma-like subsets. Clin. Cancer Res. 22, 3618–3629 (2016).
Maitra, A. & Hruban, R. H. Pancreatic cancer. Annu. Rev. Pathol. 3, 157–188 (2008).
Almoguera, C. et al. Most human carcinomas of the exocrine pancreas contain mutant c-K-ras genes. Cell 53, 549–554 (1988).
Hingorani, S. R. et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell 4, 437–450 (2003).
De La O, J.-P. et al. Notch and Kras reprogram pancreatic acinar cells to ductal intraepithelial neoplasia. Proc. Natl Acad. Sci. USA 105, 18907–18912 (2008).
Miyamoto, Y. et al. Notch mediates TGFα-induced changes in epithelial differentiation during pancreatic tumorigenesis. Cancer Cell 3, 565–576 (2003).
Mazur, P. K. et al. Notch2 is required for progression of pancreatic intraepithelial neoplasia and development of pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 107, 13438–13443 (2010).
Plentz, R. et al. Inhibition of gamma-secretase activity inhibits tumor progression in a mouse model of pancreatic ductal adenocarcinoma. Gastroenterology 136, 1741–1749 (2009).
Maniati, E. et al. Crosstalk between the canonical NF-κB and Notch signaling pathways inhibits Ppargamma expression and promotes pancreatic cancer progression in mice. J. Clin. Invest. 121, 4685–4699 (2011).
Cook, N. et al. Gamma secretase inhibition promotes hypoxic necrosis in mouse pancreatic ductal adenocarcinoma. J. Exp. Med. 209, 437–444 (2012).
Hanlon, L. et al. Notch1 functions as a tumor suppressor in a model of K-ras-induced pancreatic ductal adenocarcinoma. Cancer Res. 70, 4280–4286 (2010).
Oncomed. Oncomed provides update on tarextumab phase 2 pancreatic cancer ALPINE trial. GlobeNewswire https://globenewswire.com/news-release/2016/01/25/804294/0/en/OncoMed-Provides-Update-on-Tarextumab-Phase-2-Pancreatic-Cancer-ALPINE-Trial.html (2016).
Nakhai, H. et al. Conditional ablation of Notch signaling in pancreatic development. Development 135, 2757–2765 (2008).
Avila, J. L. & Kissil, J. L. Notch signaling in pancreatic cancer: oncogene or tumor suppressor? Trends Mol. Med. 19, 320–327 (2013).
Bilous, N. I., Abramenko, I. V., Chumak, A. A., Dyagil, I. S. & Martina, Z. V. Detection of NOTCH1 c.7541_7542delCT mutation in chronic lymphocytic leukemia using conventional and real-time polymerase chain reaction. Exp. Oncol. 38, 112–116 (2016).
Lobry, C. et al. Notch pathway activation targets AML-initiating cell homeostasis and differentiation. J. Exp. Med. 210, 301–319 (2013).
Klinakis, A. et al. A novel tumour-suppressor function for the Notch pathway in myeloid leukaemia. Nature 473, 230–233 (2011).
Kode, A. et al. Leukaemogenesis induced by an activating beta-catenin mutation in osteoblasts. Nature 506, 240–244 (2014).
Dill, M. T. et al. Disruption of Notch1 induces vascular remodeling, intussusceptive angiogenesis, and angiosarcomas in livers of mice. Gastroenterology 142, 967–977 (2012).
Kuhnert, F., Kirshner, J. R. & Thurston, G. Dll4–Notch signaling as a therapeutic target in tumor angiogenesis. Vasc. Cell 3, 20 (2011).
Ridgway, J. et al. Inhibition of Dll4 signalling inhibits tumour growth by deregulating angiogenesis. Nature 444, 1083–1087 (2006).
Yugawa, T. et al. DeltaNp63alpha repression of the Notch1 gene supports the proliferative capacity of normal human keratinocytes and cervical cancer cells. Cancer Res. 70, 4034–4044 (2010).
Chidgey, M. et al. Mice lacking desmocollin 1 show epidermal fragility accompanied by barrier defects and abnormal differentiation. J. Cell Biol. 155, 821–832 (2001).
Dumortier, A. et al. Atopic dermatitis-like disease and associated lethal myeloproliferative disorder arise from loss of Notch signaling in the murine skin. PLoS ONE 5, e9258 (2010).
Ezhkova, E. et al. EZH1 and EZH2 cogovern histone H3K27 trimethylation and are essential for hair follicle homeostasis and wound repair. Genes Dev. 25, 485–498 (2011).
Tan, M. J. et al. Cutaneous beta-human papillomavirus E6 proteins bind Mastermind-like coactivators and repress Notch signaling. Proc. Natl Acad. Sci. USA 109, E1473–E1480 (2012).
Meyers, J. M., Spangle, J. M. & Munger, K. The human papillomavirus type 8 E6 protein interferes with NOTCH activation during keratinocyte differentiation. J. Virol. 87, 4762–4767 (2013).
Aldabagh, B., Angelas, J. G., Cardones, A. R. & Arron, S. T. Cutaneous squamous cell carcinoma and human papillomavirus: is there an association? Dermatol. Surg. 39, 1–23 (2013).
Connolly, K., Manders, P., Earls, P. & Epstein, R. J. Papillomavirus-associated squamous skin cancers following transplant immunosuppression: one Notch closer to control. Cancer Treat. Rev. 40, 205–214 (2014).
Lefort, K. et al. Notch1 is a p53 target gene involved in human keratinocyte tumor suppression through negative regulation of ROCK1/2 and MRCKα kinases. Genes Dev. 21, 562–577 (2007).
Parsa, R., Yang, A., McKeon, F. & Green, H. Association of p63 with proliferative potential in normal and neoplastic human keratinocytes. J. Invest. Dermatol. 113, 1099–1105 (1999).
Rocco, J. W., Leong, C. O., Kuperwasser, N., DeYoung, M. P. & Ellisen, L. W. p63 mediates survival in squamous cell carcinoma by suppression of p73-dependent apoptosis. Cancer Cell 9, 45–56 (2006).
Keyes, W. M. et al. DeltaNp63alpha is an oncogene that targets chromatin remodeler Lsh to drive skin stem cell proliferation and tumorigenesis. Cell Stem Cell 8, 164–176 (2011).
Xu, C. et al. Loss of Lkb1 and Pten leads to lung squamous cell carcinoma with elevated PD-L1 expression. Cancer Cell 25, 590–604 (2014).
Yang, A. et al. p63, a p53 homolog at 3q27-29, encodes multiple products with transactivating, death-inducing, and dominant-negative activities. Mol. Cell 2, 305–316 (1998).
Senoo, M., Pinto, F., Crum, C. P. & McKeon, F. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell 129, 523–536 (2007).
Nguyen, B. C. et al. Cross-regulation between Notch and p63 in keratinocyte commitment to differentiation. Genes Dev. 20, 1028–1042 (2006).
Eferl, R. & Wagner, E. F. AP-1: a double-edged sword in tumorigenesis. Nat. Rev. Cancer 3, 859–868 (2003).
Guinea-Viniegra, J. et al. Differentiation-induced skin cancer suppression by FOS, 53, and TACE/ADAM17. J. Clin. Invest. 122, 2898–2910 (2012). The findings of this study demonstrated that FOS and p53 prevented skin cancer development by inducing Notch activity through promoting ADAM17 expression.
Dotto, G. P. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat. Rev. Cancer 9, 587–595 (2009).
Kolev, V. et al. EGFR signalling as a negative regulator of Notch1 gene transcription and function in proliferating keratinocytes and cancer. Nat. Cell Biol. 10, 902–911 (2008).
Zanconato, F. et al. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nat. Cell Biol. 17, 1218–1227 (2015).
Murthy, A. et al. Notch activation by the metalloproteinase ADAM17 regulates myeloproliferation and atopic barrier immunity by suppressing epithelial cytokine synthesis. Immunity 36, 105–119 (2012).
Nowell, C. S. et al. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 18, 168–180 (2016). This study revealed how loss of Notch signalling in regenerating epithelia induces aberrant cell fate in stem and progenitor cells indirectly by altering the mechanical properties of the tissue stroma as a consequence of chronic inflammation.
Demehri, S. et al. Notch-deficient skin induces a lethal systemic B-lymphoproliferative disorder by secreting TSLP, a sentinel for epidermal integrity. PLoS Biol. 6, e123 (2008). References 95, 114 and 116 all used mouse models to show that many of the pathological consequences of loss of Notch signalling in the epidermis may in part be due to aberrant inflammatory responses in the skin.
Rangarajan, A. et al. Notch signaling is a direct determinant of keratinocyte growth arrest and entry into differentiation. EMBO J. 20, 3427–3436 (2001).
Restivo, G. et al. IRF6 is a mediator of Notch pro-differentiation and tumour suppressive function in keratinocytes. EMBO J. 30, 4571–4585 (2011).
Vauclair, S. et al. Corneal epithelial cell fate is maintained during repair by Notch1 signaling via the regulation of vitamin A metabolism. Dev. Cell 13, 242–253 (2007).
Collins, C. A. & Watt, F. M. Dynamic regulation of retinoic acid-binding proteins in developing, adult and neoplastic skin reveals roles for beta-catenin and Notch signalling. Dev. Biol. 324, 55–67 (2008).
Saitou, M. et al. Inhibition of skin development by targeted expression of a dominant-negative retinoic acid receptor. Nature 374, 159–162 (1995).
Syed, Z. et al. All-trans retinoic acid suppresses Stat3 signaling during skin carcinogenesis. Cancer Prev. Res. 2, 903–911 (2009).
Andersen, J. et al. A transcriptional mechanism integrating inputs from extracellular signals to activate hippocampal stem cells. Neuron 83, 1085–1097 (2014).
Imayoshi, I. et al. Oscillatory control of factors determining multipotency and fate in mouse neural progenitors. Science 342, 1203–1208 (2013).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Coussens, L. M. & Werb, Z. Inflammation and cancer. Nature 420, 860–867 (2002).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Mantovani, A., Allavena, P., Sica, A. & Balkwill, F. Cancer-related inflammation. Nature 454, 436–444 (2008).
Demehri, S., Turkoz, A. & Kopan, R. Epidermal Notch1 loss promotes skin tumorigenesis by impacting the stromal microenvironment. Cancer Cell 16, 55–66 (2009). This work importantly demonstrated a non-cell-autonomous mechanism of Notch-mediated tumour suppression.
Hu, B. et al. Multifocal epithelial tumors and field cancerization from loss of mesenchymal CSL signaling. Cell 149, 1207–1220 (2012).
Demehri, S. et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 22, 494–505 (2012).
Di Piazza, M., Nowell, C. S., Koch, U., Durham, A. D. & Radtke, F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 22, 479–493 (2012). References 131 and 132 demonstrated how aberrant cytokine activity in Notch-deficient epidermal epithelial cells influenced pro-tumorigenic versus antitumorigenic inflammation in the skin.
Espinosa, L. et al. The Notch/Hes1 pathway sustains NF-kappaB activation through CYLD repression in T cell leukemia. Cancer Cell 18, 268–281 (2010).
Coombs, C. C., Tavakkoli, M. & Tallman, M. S. Acute promyelocytic leukemia: where did we start, where are we now, and the future. Blood Cancer J. 5, e304 (2015).
Yu, X. M., Phan, T., Patel, P. N., Jaskula-Sztul, R. & Chen, H. Chrysin activates Notch1 signaling and suppresses tumor growth of anaplastic thyroid carcinoma in vitro and in vivo. Cancer 119, 774–781 (2013).
Patel, P. N., Yu, X. M., Jaskula-Sztul, R. & Chen, H. Hesperetin activates the Notch1 signaling cascade, causes apoptosis, and induces cellular differentiation in anaplastic thyroid cancer. Ann. Surg. Oncol. 21 (Suppl. 4), S497–S504 (2014).
Greenblatt, D. Y. et al. Valproic acid activates notch-1 signaling and regulates the neuroendocrine phenotype in carcinoid cancer cells. Oncologist 12, 942–951 (2007).
Mohammed, T. A. et al. A pilot phase II study of valproic acid for treatment of low-grade neuroendocrine carcinoma. Oncologist 16, 835–843 (2011).
Li, K. et al. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J. Biol. Chem. 283, 8046–8054 (2008).
Wu, Y. et al. Therapeutic antibody targeting of individual Notch receptors. Nature 464, 1052–1057 (2010).
Chiu, M. L. & Gilliland, G. L. Engineering antibody therapeutics. Curr. Opin. Struct. Biol. 38, 163–173 (2016).
Auderset, F., Coutaz, M. & Tacchini-Cottier, F. The role of Notch in the differentiation of CD4+ T helper cells. Curr. Top. Microbiol. Immunol. 360, 115–134 (2012).
Amsen, D. et al. Instruction of distinct CD4 T helper cell fates by different notch ligands on antigen-presenting cells. Cell 117, 515–526 (2004).
Amsen, D., Antov, A. & Flavell, R. A. The different faces of Notch in T-helper-cell differentiation. Nat. Rev. Immunol. 9, 116–124 (2009).
Mosmann, T. R. & Coffman, R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu. Rev. Immunol. 7, 145–173 (1989).
Vinay, D. S. et al. Immune evasion in cancer: mechanistic basis and therapeutic strategies. Semin. Cancer Biol. 35, 185–198 (2015).
Ellisen, L. W. et al. TAN-1, the human homolog of the Drosophila notch gene, is broken by chromosomal translocations in T lymphoblastic neoplasms. Cell 66, 649–661 (1991).
Reynolds, T. C., Smith, S. D. & Sklar, J. Analysis of DNA surrounding the breakpoints of chromosomal translocations involving the beta T cell receptor gene in human lymphoblastic neoplasms. Cell 50, 107–117 (1987).
Capobianco, A. J., Zagouras, P., Blaumueller, C. M., Artavanis-Tsakonas, S. & Bishop, J. M. Neoplastic transformation by truncated alleles of human NOTCH1/TAN1 and NOTCH2. Mol. Cell. Biol. 17, 6265–6273 (1997).
Pear, W. S. et al. Exclusive development of T cell neoplasms in mice transplanted with bone marrow expressing activated Notch alleles. J. Exp. Med. 183, 2283–2291 (1996).
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Di Ianni, M. et al. A new genetic lesion in B-CLL: a NOTCH1 PEST domain mutation. Br. J. Haematol. 146, 689–691 (2009).
Fabbri, G. et al. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J. Exp. Med. 208, 1389–1401 (2011).
Puente, X. S. et al. Whole-genome sequencing identifies recurrent mutations in chronic lymphocytic leukaemia. Nature 475, 101–105 (2011).
Sportoletti, P. et al. NOTCH1 PEST domain mutation is an adverse prognostic factor in B-CLL. Br. J. Haematol. 151, 404–406 (2010).
Kridel, R. et al. Whole transcriptome sequencing reveals recurrent NOTCH1 mutations in mantle cell lymphoma. Blood 119, 1963–1971 (2012).
Rossi, D. et al. The coding genome of splenic marginal zone lymphoma: activation of NOTCH2 and other pathways regulating marginal zone development. J. Exp. Med. 209, 1537–1551 (2012).
Kiel, M. J. et al. Whole-genome sequencing identifies recurrent somatic NOTCH2 mutations in splenic marginal zone lymphoma. J. Exp. Med. 209, 1553–1565 (2012).
Gallahan, D., Kozak, C. & Callahan, R. A new common integration region (int-3) for mouse mammary tumor virus on mouse chromosome 17. J. Virol. 61, 218–220 (1987).
Reedijk, M. et al. High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res. 65, 8530–8537 (2005).
Reedijk, M. et al. JAG1 expression is associated with a basal phenotype and recurrence in lymph node-negative breast cancer. Breast Cancer Res. Treat. 111, 439–448 (2008).
Pece, S. et al. Loss of negative regulation by Numb over Notch is relevant to human breast carcinogenesis. J. Cell Biol. 167, 215–221 (2004).
Robinson, D. R. et al. Functionally recurrent rearrangements of the MAST kinase and Notch gene families in breast cancer. Nat. Med. 17, 1646–1651 (2011).
Maraver, A. et al. Therapeutic effect of gamma-secretase inhibition in KrasG12V-driven non-small cell lung carcinoma by derepression of DUSP1 and inhibition of ERK. Cancer Cell 22, 222–234 (2012).
Koch, U. & Radtke, F. Notch signaling in solid tumors. Curr. Top. Dev. Biol. 92, 411–455 (2010).
Ranganathan, P., Weaver, K. L. & Capobianco, A. J. Notch signalling in solid tumours: a little bit of everything but not all the time. Nat. Rev. Cancer 11, 338–351 (2011).
Zender, S. et al. A critical role for notch signaling in the formation of cholangiocellular carcinomas. Cancer Cell 23, 784–795 (2013).
Acknowledgements
The work in the authors' laboratory is supported in part by the Swiss National Science Foundation and the Swiss Cancer League.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- γ-Secretase
-
A multi-protein complex mediating the proteolytic S3 cleavage of Notch receptors upon ligand binding, thereby liberating the intracellular domain of Notch. The complex consists of presenilin 1, presenilin 2, nicastrin, anterior pharynx-defective 1 (APH1) and presenilin enhancer 2 (PEN2).
- DMBA–TPA mouse model of cutaneous chemical carcinogenesis
-
A two-stage chemical skin carcinogenesis model that uses a single dose of the genotoxic carcinogen DMBA followed by multiple doses of a non-genotoxic tumour promoter, TPA.
- Field change
-
The molecular alteration of large areas or groups of cells in a tissue. The molecular aberrations acquired are usually induced by prolonged injurious events and predispose to neoplasia and carcinogenesis.
- MLL–AF9
-
An oncogene generated by the chromosomal translocation t(9;11) (p22;q23). This results in the in-frame fusion of mixed lineage leukaemia (MLL) with AF9. The transcripts produced from this fusion promote the development of myeloid leukaemias.
- Atopic dermatitis
-
An inflammatory skin disorder that is believed to be an immune hypersensitive response to environmental stimuli. Evidence supports a genetic basis for the disease.
- Metaplasia
-
A change in the cell type that populates a given tissue, resulting in abnormal architecture, morphology and function.
Rights and permissions
About this article
Cite this article
Nowell, C., Radtke, F. Notch as a tumour suppressor. Nat Rev Cancer 17, 145–159 (2017). https://doi.org/10.1038/nrc.2016.145
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.145