Key Points
-
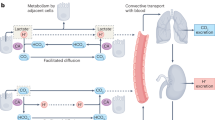
In contrast to healthy tissues, the extracellular pH of tumours is generally acidic, while the intracellular pH is slightly alkaline.
-
Exacerbated glycolysis and respiration through hydration of CO2 contribute to the release of H+ ions in the tumour microenvironment, making gradients of acidosis and hypoxia non-overlapping.
-
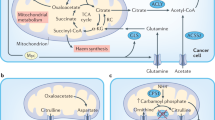
Tumour acidosis induces a shift from HIF1α-driven glycolytic metabolism towards the metabolism of glutamine and lipids as preferred sources of energy and biosynthetic intermediates.
-
Adaptation of cancer cells to acidosis requires transcriptional (for example, HIF2α induction), post-translational (for example, changes in protein acetylation) and morphological alterations (for example, mitochondria elongation with an increase in cristae numbers).
-
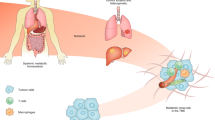
Acidosis-driven tumour progression is promoted by a reduction in immunosurveillance and changes in lysosome biology that support invasiveness and autophagy.
-
Tumour acidosis can be targeted by drugs interfering with H+ or bicarbonate transporters, neutralized by systemic buffer administration or exploited using pH-sensitive drug-delivery systems.
Abstract
The high metabolic demand of cancer cells leads to an accumulation of H+ ions in the tumour microenvironment. The disorganized tumour vasculature prevents an efficient wash-out of H+ ions released into the extracellular medium but also favours the development of tumour hypoxic regions associated with a shift towards glycolytic metabolism. Under hypoxia, the final balance of glycolysis, including breakdown of generated ATP, is the production of lactate and a stoichiometric amount of H+ ions. Another major source of H+ ions results from hydration of CO2 produced in the more oxidative tumour areas. All of these events occur at high rates in tumours to fulfil bioenergetic and biosynthetic needs. This Review summarizes the current understanding of how H+-generating metabolic processes segregate within tumours according to the distance from blood vessels and inversely how ambient acidosis influences tumour metabolism, reducing glycolysis while promoting mitochondrial activity. The Review also presents novel insights supporting the participation of acidosis in cancer progression via stimulation of autophagy and immunosuppression. Finally, recent advances in the different therapeutic modalities aiming to either block pH-regulatory systems or exploit acidosis will be discussed.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Nakazawa, M. S., Keith, B. & Simon, M. C. Oxygen availability and metabolic adaptations. Nat. Rev. Cancer 16, 663–673 (2016). This review summarizes how metabolic adaptations are integrated in hypoxic tumour cells and their role in disease progression.
Sennino, B. & McDonald, D. M. Controlling escape from angiogenesis inhibitors. Nat. Rev. Cancer 12, 699–709 (2012).
Bertout, J. A., Patel, S. A. & Simon, M. C. The impact of O2 availability on human cancer. Nat. Rev. Cancer 8, 967–975 (2008).
Srivastava, J., Barber, D. L. & Jacobson, M. P. Intracellular pH sensors: design principles and functional significance. Physiol. (Bethesda) 22, 30–39 (2007).
Neri, D. & Supuran, C. T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 10, 767–777 (2011).
Michiels, C., Tellier, C. & Feron, O. Cycling hypoxia: a key feature of the tumor microenvironment. Biochim. Biophys. Acta 1866, 76–86 (2016).
Secomb, T. W., Dewhirst, M. W. & Pries, A. R. Structural adaptation of normal and tumour vascular networks. Bas. Clin. Pharmacol. Toxicol. 110, 63–69 (2012).
Vaupel, P. & Mayer, A. Hypoxia in tumors: pathogenesis-related classification, characterization of hypoxia subtypes, and associated biological and clinical implications. Adv. Exp. Med. Biol. 812, 19–24 (2014).
Corbet, C. & Feron, O. Cancer cell metabolism and mitochondria: nutrient plasticity for TCA cycle fueling. Biochim. Biophys. Acta 1868, 7–15 (2017).
Draoui, N. & Feron, O. Lactate shuttles at a glance: from physiological paradigms to anti-cancer treatments. Dis. Model. Mech. 4, 727–732 (2011).
Vander Heiden, M. G., Cantley, L. C. & Thompson, C. B. Understanding the Warburg effect: the metabolic requirements of cell proliferation. Science 324, 1029–1033 (2009).
Zu, X. L. & Guppy, M. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 313, 459–465 (2004).
Webb, B. A., Chimenti, M., Jacobson, M. P. & Barber, D. L. Dysregulated pH: a perfect storm for cancer progression. Nat. Rev. Cancer 11, 671–677 (2011).
Lagadic-Gossmann, D., Huc, L. & Lecureur, V. Alterations of intracellular pH homeostasis in apoptosis: origins and roles. Cell Death Differ. 11, 953–961 (2004).
Hulikova, A., Harris, A. L., Vaughan-Jones, R. D. & Swietach, P. Regulation of intracellular pH in cancer cell lines under normoxia and hypoxia. J. Cell. Physiol. 228, 743–752 (2013).
Fukuda, R. et al. HIF-1 regulates cytochrome oxidase subunits to optimize efficiency of respiration in hypoxic cells. Cell 129, 111–122 (2007). This study describes how the COX4 isoform switch acts as a homeostatic response that optimizes the efficiency of respiration at different O 2 concentrations.
Boidot, R. et al. Regulation of monocarboxylate transporter MCT1 expression by p53 mediates inward and outward lactate fluxes in tumors. Cancer Res. 72, 939–948 (2012).
Khacho, M. et al. Acidosis overrides oxygen deprivation to maintain mitochondrial function and cell survival. Nat. Commun. 5, 3550 (2014). This study reports how mild acidosis can change mitochondrial morphology to preserve efficient ATP production regardless of O 2 levels.
Mookerjee, S. A., Goncalves, R. L., Gerencser, A. A., Nicholls, D. G. & Brand, M. D. The contributions of respiration and glycolysis to extracellular acid production. Biochim. Biophys. Acta 1847, 171–181 (2015).
Supuran, C. T. Carbonic anhydrases: novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 7, 168–181 (2008).
Musa-Aziz, R., Chen, L. M., Pelletier, M. F. & Boron, W. F. Relative CO2/NH3 selectivities of AQP1, AQP4, AQP5, AmtB, and RhAG. Proc. Natl Acad. Sci. USA 106, 5406–5411 (2009).
Hulikova, A. & Swietach, P. Rapid CO2 permeation across biological membranes: implications for CO2 venting from tissue. FASEB J. 28, 2762–2774 (2014).
Svastova, E. et al. Hypoxia activates the capacity of tumor-associated carbonic anhydrase IX to acidify extracellular pH. FEBS Lett. 577, 439–445 (2004).
Swietach, P., Vaughan-Jones, R. D., Harris, A. L. & Hulikova, A. The chemistry, physiology and pathology of pH in cancer. Phil. Trans. R. Soc. B 369, 20130099 (2014).
Hulikova, A., Vaughan-Jones, R. D. & Swietach, P. Dual role of CO2/HCO3− buffer in the regulation of intracellular pH of three-dimensional tumor growths. J. Biol. Chem. 286, 13815–13826 (2011).
Swietach, P., Hulikova, A., Vaughan-Jones, R. D. & Harris, A. L. New insights into the physiological role of carbonic anhydrase IX in tumour pH regulation. Oncogene 29, 6509–6521 (2010).
Becker, H. M., Klier, M., Schuler, C., McKenna, R. & Deitmer, J. W. Intramolecular proton shuttle supports not only catalytic but also noncatalytic function of carbonic anhydrase II. Proc. Natl Acad. Sci. USA 108, 3071–3076 (2011).
Deitmer, J. W. & Becker, H. M. Transport metabolons with carbonic anhydrases. Front. Physiol. 4, 291 (2013).
Klier, M., Andes, F. T., Deitmer, J. W. & Becker, H. M. Intracellular and extracellular carbonic anhydrases cooperate non-enzymatically to enhance activity of monocarboxylate transporters. J. Biol. Chem. 289, 2765–2775 (2014).
Jamali, S. et al. Hypoxia-induced carbonic anhydrase IX facilitates lactate flux in human breast cancer cells by non-catalytic function. Sci. Rep. 5, 13605 (2015).
Helmlinger, G., Yuan, F., Dellian, M. & Jain, R. K. Interstitial pH and pO2 gradients in solid tumors in vivo: high-resolution measurements reveal a lack of correlation. Nat. Med. 3, 177–182 (1997).
Vaupel, P. W., Frinak, S. & Bicher, H. I. Heterogeneous oxygen partial pressure and pH distribution in C3H mouse mammary adenocarcinoma. Cancer Res. 41, 2008–2013 (1981). References 31 and 32 reported the first in vivo evidence that pH and p O2 profiles do not overlap in mouse tumour models.
Bittner, M. I. et al. Analysis of relation between hypoxia PET imaging and tissue-based biomarkers during head and neck radiochemotherapy. Acta Oncol. 55, 1299–1304 (2016).
Le, Q. T. et al. Expression and prognostic significance of a panel of tissue hypoxia markers in head-and-neck squamous cell carcinomas. Int. J. Radiat. Oncol. Biol. Phys. 69, 167–175 (2007).
Rademakers, S. E., Lok, J., van der Kogel, A. J., Bussink, J. & Kaanders, J. H. Metabolic markers in relation to hypoxia; staining patterns and colocalization of pimonidazole, HIF-1α, CAIX, LDH-5, GLUT-1, MCT1 and MCT4. BMC Cancer 11, 167 (2011).
Helmlinger, G., Sckell, A., Dellian, M., Forbes, N. S. & Jain, R. K. Acid production in glycolysis-impaired tumors provides new insights into tumor metabolism. Clin. Cancer Res. 8, 1284–1291 (2002).
Newell, K., Franchi, A., Pouyssegur, J. & Tannock, I. Studies with glycolysis-deficient cells suggest that production of lactic acid is not the only cause of tumor acidity. Proc. Natl Acad. Sci. USA 90, 1127–1131 (1993). This study documents that production of lactic acid via glycolysis is not the only mechanism responsible for the development of an acidic environment in RAS-overexpressing fibroblast-derived tumours.
Yamagata, M., Hasuda, K., Stamato, T. & Tannock, I. F. The contribution of lactic acid to acidification of tumours: studies of variant cells lacking lactate dehydrogenase. Br. J. Cancer 77, 1726–1731 (1998).
Hulikova, A. et al. Stromal uptake and transmission of acid is a pathway for venting cancer cell-generated acid. Proc. Natl Acad. Sci. USA 113, E5344–E5353 (2016).
Corbet, C. et al. Acidosis drives the reprogramming of fatty acid metabolism in cancer cells through changes in mitochondrial and histone acetylation. Cell Metab. 24, 311–323 (2016).
Corbet, C. et al. The SIRT1/HIF2α axis drives reductive glutamine metabolism under chronic acidosis and alters tumor response to therapy. Cancer Res. 74, 5507–5519 (2014). References 40 and 41 describe how the study of cancer cells chronically adapted to acidosis led to the discovery that cancer cells could simultaneously synthesize fatty acids from glutamine and degrade exogenous fatty acids in the mitochondria.
Filatova, A. et al. Acidosis acts through HSP90 in a PHD/VHL-independent manner to promote HIF function and stem cell maintenance in glioma. Cancer Res. 76, 5845–5856 (2016).
Nadtochiy, S. M. et al. Acidic pH is a metabolic switch for 2-Hydroxyglutarate generation and signaling. J. Biol. Chem. 291, 20188–20197 (2016).
Kondo, A. et al. Extracellular acidic pH activates the sterol regulatory element-binding protein 2 to promote tumor progression. Cell Rep. 18, 2228–2242 (2017).
Mekhail, K., Gunaratnam, L., Bonicalzi, M. E. & Lee, S. HIF activation by pH-dependent nucleolar sequestration of VHL. Nat. Cell Biol. 6, 642–647 (2004).
Hjelmeland, A. B. et al. Acidic stress promotes a glioma stem cell phenotype. Cell Death Differ. 18, 829–840 (2011).
Tang, X. et al. Functional interaction between responses to lactic acidosis and hypoxia regulates genomic transcriptional outputs. Cancer Res. 72, 491–502 (2012).
Chen, J. L. et al. The genomic analysis of lactic acidosis and acidosis response in human cancers. PLoS Genet. 4, e1000293 (2008).
Dioum, E. M. et al. Regulation of hypoxia-inducible factor 2α signaling by the stress-responsive deacetylase sirtuin 1. Science 324, 1289–1293 (2009).
Lim, J. H. et al. Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol. Cell 38, 864–878 (2010).
Chen, J. L. et al. Lactic acidosis triggers starvation response with paradoxical induction of TXNIP through MondoA. PLoS Genet. 6, e1001093 (2010).
Parikh, H. et al. TXNIP regulates peripheral glucose metabolism in humans. PLoS Med. 4, e158 (2007).
Glitsch, M. Protons and Ca2+: ionic allies in tumor progression? Physiol. (Bethesda) 26, 252–265 (2011).
Ludwig, M. G. et al. Proton-sensing G-protein-coupled receptors. Nature 425, 93–98 (2003).
Damaghi, M., Wojtkowiak, J. W. & Gillies, R. J. pH sensing and regulation in cancer. Front. Physiol. 4, 370 (2013).
Gupta, S. C., Singh, R., Pochampally, R., Watabe, K. & Mo, Y. Y. Acidosis promotes invasiveness of breast cancer cells through ROS-AKT-NF-κB pathway. Oncotarget 5, 12070–12082 (2014).
Chen, B., Liu, J., Ho, T. T., Ding, X. & Mo, Y. Y. ERK-mediated NF-κB activation through ASIC1 in response to acidosis. Oncogenesis 5, e279 (2016).
DiGiammarino, E. L. et al. A novel mechanism of tumorigenesis involving pH-dependent destabilization of a mutant p53 tetramer. Nat. Struct. Biol. 9, 12–16 (2002).
Sonveaux, P. et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J. Clin. Invest. 118, 3930–3942 (2008). This study reported for the first time to our knowledge the lactate-based metabolic symbiosis between glycolytic and oxidative cancer cells.
Vegran, F., Boidot, R., Michiels, C., Sonveaux, P. & Feron, O. Lactate influx through the endothelial cell monocarboxylate transporter MCT1 supports an NF-κB/IL-8 pathway that drives tumor angiogenesis. Cancer Res. 71, 2550–2560 (2011).
Allen, E. et al. Metabolic symbiosis enables adaptive resistance to anti-angiogenic therapy that is dependent on mTOR signaling. Cell Rep. 15, 1144–1160 (2016).
Jimenez-Valerio, G. et al. Resistance to antiangiogenic therapies by metabolic symbiosis in renal cell carcinoma PDX models and patients. Cell Rep. 15, 1134–1143 (2016).
Pisarsky, L. et al. Targeting metabolic symbiosis to overcome resistance to anti-angiogenic therapy. Cell Rep. 15, 1161–1174 (2016).
Feron, O. Pyruvate into lactate and back: from the Warburg effect to symbiotic energy fuel exchange in cancer cells. Radiother. Oncol. 92, 329–333 (2009).
Doherty, J. R. & Cleveland, J. L. Targeting lactate metabolism for cancer therapeutics. J. Clin. Invest. 123, 3685–3692 (2013).
Marchiq, I. & Pouyssegur, J. Hypoxia, cancer metabolism and the therapeutic benefit of targeting lactate/H+ symporters. J. Mol. Med. (Berl.) 94, 155–171 (2016).
Sun, R. C. & Denko, N. C. Hypoxic regulation of glutamine metabolism through HIF1 and SIAH2 supports lipid synthesis that is necessary for tumor growth. Cell Metab. 19, 285–292 (2014).
Wise, D. R. et al. Hypoxia promotes isocitrate dehydrogenase-dependent carboxylation of alpha-ketoglutarate to citrate to support cell growth and viability. Proc. Natl Acad. Sci. USA 108, 19611–19616 (2011).
Metallo, C. M. et al. Reductive glutamine metabolism by IDH1 mediates lipogenesis under hypoxia. Nature 481, 380–384 (2011).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Lamonte, G. et al. Acidosis induces reprogramming of cellular metabolism to mitigate oxidative stress. Cancer Metab. 1, 23 (2013).
Peppicelli, S., Bianchini, F. & Calorini, L. Extracellular acidity, a “reappreciated” trait of tumor environment driving malignancy: perspectives in diagnosis and therapy. Cancer Metastasis Rev. 33, 823–832 (2014).
Parks, S. K., Chiche, J. & Pouyssegur, J. Disrupting proton dynamics and energy metabolism for cancer therapy. Nat. Rev. Cancer 13, 611–623 (2013).
Mason, S. D. & Joyce, J. A. Proteolytic networks in cancer. Trends Cell Biol. 21, 228–237 (2011).
Gatenby, R. A., Gawlinski, E. T., Gmitro, A. F., Kaylor, B. & Gillies, R. J. Acid-mediated tumor invasion: a multidisciplinary study. Cancer Res. 66, 5216–5223 (2006).
Robertson-Tessi, M., Gillies, R. J., Gatenby, R. A. & Anderson, A. R. Impact of metabolic heterogeneity on tumor growth, invasion, and treatment outcomes. Cancer Res. 75, 1567–1579 (2015).
Mohamed, M. M. & Sloane, B. F. Cysteine cathepsins: multifunctional enzymes in cancer. Nat. Rev. Cancer 6, 764–775 (2006).
Estrella, V. et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 73, 1524–1535 (2013). This study describes the monitoring of acid- mediated tumour invasion over time using intravital microscopy.
Lee, S., Jilani, S. M., Nikolova, G. V., Carpizo, D. & Iruela-Arispe, M. L. Processing of VEGF-A by matrix metalloproteinases regulates bioavailability and vascular patterning in tumors. J. Cell Biol. 169, 681–691 (2005).
Fukumura, D. et al. Hypoxia and acidosis independently up-regulate vascular endothelial growth factor transcription in brain tumors in vivo. Cancer Res. 61, 6020–6024 (2001).
Avnet, S. et al. Cancer-associated mesenchymal stroma fosters the stemness of osteosarcoma cells in response to intratumoral acidosis via NF-kappaB activation. Int. J. Cancer 140, 1331–1345 (2017).
Peppicelli, S. et al. Extracellular acidity strengthens mesenchymal stem cells to promote melanoma progression. Cell Cycle 14, 3088–3100 (2015).
Rothberg, J. M. et al. Acid-mediated tumor proteolysis: contribution of cysteine cathepsins. Neoplasia 15, 1125–1137 (2013).
Heuser, J. Changes in lysosome shape and distribution correlated with changes in cytoplasmic pH. J. Cell Biol. 108, 855–864 (1989).
Glunde, K. et al. Extracellular acidification alters lysosomal trafficking in human breast cancer cells. Neoplasia 5, 533–545 (2003).
Steffan, J. J., Snider, J. L., Skalli, O., Welbourne, T. & Cardelli, J. A. Na+/H+ exchangers and RhoA regulate acidic extracellular pH-induced lysosome trafficking in prostate cancer cells. Traffic 10, 737–753 (2009).
Sennoune, S. R. et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am. J. Physiol. Cell Physiol. 286, C1443–C1452 (2004).
Damaghi, M. et al. Chronic acidosis in the tumour microenvironment selects for overexpression of LAMP2 in the plasma membrane. Nat. Commun. 6, 8752 (2015).
Wojtkowiak, J. W. et al. Chronic autophagy is a cellular adaptation to tumor acidic pH microenvironments. Cancer Res. 72, 3938–3947 (2012).
Robey, I. F. et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 69, 2260–2268 (2009).
Marino, M. L. et al. Autophagy is a protective mechanism for human melanoma cells under acidic stress. J. Biol. Chem. 287, 30664–30676 (2012).
Balgi, A. D. et al. Regulation of mTORC1 signaling by pH. PLoS ONE 6, e21549 (2011).
Brisson, L. et al. Lactate dehydrogenase B controls lysosome activity and autophagy in cancer. Cancer Cell 30, 418–431 (2016).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Dietl, K. et al. Lactic acid and acidification inhibit TNF secretion and glycolysis of human monocytes. J. Immunol. 184, 1200–1209 (2010).
Brand, A. et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. 24, 657–671 (2016).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). This study describes how alterations in tumour metabolic preferences may impact the ability of T cells to control tumour growth.
Spugnini, E. & Fais, S. Proton pump inhibition and cancer therapeutics: a specific tumor targeting or it is a phenomenon secondary to a systemic buffering? Semin. Cancer Biol. (2017).
Fais, S., Venturi, G. & Gatenby, B. Microenvironmental acidosis in carcinogenesis and metastases: new strategies in prevention and therapy. Cancer Metastasis Rev. 33, 1095–1108 (2014).
Aronson, J. K. Inhibiting the proton pump: mechanisms, benefits, harms, and questions. BMC Med. 14, 172 (2016).
Olbe, L., Carlsson, E. & Lindberg, P. A proton-pump inhibitor expedition: the case histories of omeprazole and esomeprazole. Nat. Rev. Drug Discov. 2, 132–139 (2003).
Mattsson, J. P., Vaananen, K., Wallmark, B. & Lorentzon, P. Omeprazole and bafilomycin, two proton pump inhibitors: differentiation of their effects on gastric, kidney and bone H+-translocating ATPases. Biochim. Biophys. Acta 1065, 261–268 (1991).
De Milito, A. et al. Proton pump inhibitors induce apoptosis of human B-cell tumors through a caspase-independent mechanism involving reactive oxygen species. Cancer Res. 67, 5408–5417 (2007).
Perut, F. et al. V-ATPase as an effective therapeutic target for sarcomas. Exp. Cell Res. 320, 21–32 (2014).
Luciani, F. et al. Effect of proton pump inhibitor pretreatment on resistance of solid tumors to cytotoxic drugs. J. Natl Cancer Inst. 96, 1702–1713 (2004).
Taylor, S. et al. Microenvironment acidity as a major determinant of tumor chemoresistance: Proton pump inhibitors (PPIs) as a novel therapeutic approach. Drug Resist. Updat. 23, 69–78 (2015).
Yuan, N. et al. Bafilomycin A1 targets both autophagy and apoptosis pathways in pediatric B-cell acute lymphoblastic leukemia. Haematologica 100, 345–356 (2015).
Yan, Y. et al. Bafilomycin A1 induces caspase-independent cell death in hepatocellular carcinoma cells via targeting of autophagy and MAPK pathways. Sci. Rep. 6, 37052 (2016).
Chiche, J. et al. Hypoxia-inducible carbonic anhydrase IX and XII promote tumor cell growth by counteracting acidosis through the regulation of the intracellular pH. Cancer Res. 69, 358–368 (2009).
Watson, P. H. et al. Carbonic anhydrase XII is a marker of good prognosis in invasive breast carcinoma. Br. J. Cancer 88, 1065–1070 (2003).
Lloyd, M. C. et al. Darwinian dynamics of intratumoral heterogeneity: not solely random mutations but also variable environmental selection forces. Cancer Res. 76, 3136–3144 (2016).
Lou, Y. et al. Targeting tumor hypoxia: suppression of breast tumor growth and metastasis by novel carbonic anhydrase IX inhibitors. Cancer Res. 71, 3364–3376 (2011).
Doyen, J., Parks, S. K., Marcie, S., Pouyssegur, J. & Chiche, J. Knock-down of hypoxia-induced carbonic anhydrases IX and XII radiosensitizes tumor cells by increasing intracellular acidosis. Front. Oncol. 2, 199 (2012).
McIntyre, A. et al. Carbonic anhydrase IX promotes tumor growth and necrosis in vivo and inhibition enhances anti-VEGF therapy. Clin. Cancer Res. 18, 3100–3111 (2012).
Petrul, H. M. et al. Therapeutic mechanism and efficacy of the antibody-drug conjugate BAY 79–4620 targeting human carbonic anhydrase 9. Mol. Cancer Ther. 11, 340–349 (2012).
Siebels, M. et al. A clinical phase I/II trial with the monoclonal antibody cG250 (RENCAREX®) and interferon-alpha-2a in metastatic renal cell carcinoma patients. World J. Urol. 29, 121–126 (2011).
Parkkila, S. et al. The protein tyrosine kinase inhibitors imatinib and nilotinib strongly inhibit several mammalian α-carbonic anhydrase isoforms. Bioorg. Med. Chem. Lett. 19, 4102–4106 (2009).
Weber, A. et al. Unexpected nanomolar inhibition of carbonic anhydrase by COX-2-selective celecoxib: new pharmacological opportunities due to related binding site recognition. J. Med. Chem. 47, 550–557 (2004).
Bola, B. M. et al. Inhibition of monocarboxylate transporter-1 (MCT1) by AZD3965 enhances radiosensitivity by reducing lactate transport. Mol. Cancer Ther. 13, 2805–2816 (2014).
Hong, C. S. et al. MCT1 modulates cancer cell pyruvate export and growth of tumors that co-express MCT1 and MCT4. Cell Rep. 14, 1590–1601 (2016).
Polanski, R. et al. Activity of the monocarboxylate transporter 1 inhibitor AZD3965 in small cell lung cancer. Clin. Cancer Res. 20, 926–937 (2014).
Lee, Y. et al. Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487, 443–448 (2012).
Suzuki, A. et al. Astrocyte-neuron lactate transport is required for long-term memory formation. Cell 144, 810–823 (2011).
Gottfried, E. et al. New aspects of an old drug—diclofenac targets MYC and glucose metabolism in tumor cells. PLoS ONE 8, e66987 (2013).
Tannock, I. F. & Rotin, D. Acid pH in tumors and its potential for therapeutic exploitation. Cancer Res. 49, 4373–4384 (1989).
Lagarde, A. E., Franchi, A. J., Paris, S. & Pouyssegur, J. M. Effect of mutations affecting Na+: H+ antiport activity on tumorigenic potential of hamster lung fibroblasts. J. Cell. Biochem. 36, 249–260 (1988).
Parks, S. K., Cormerais, Y., Durivault, J. & Pouyssegur, J. Genetic disruption of the pHi-regulating proteins Na+/H+ exchanger 1 (SLC9A1) and carbonic anhydrase 9 severely reduces growth of colon cancer cells. Oncotarget 8, 10225–10237 (2017).
Pouyssegur, J., Sardet, C., Franchi, A., L'Allemain, G. & Paris, S. A specific mutation abolishing Na+/H+ antiport activity in hamster fibroblasts precludes growth at neutral and acidic pH. Proc. Natl Acad. Sci. USA 81, 4833–4837 (1984).
Rich, I. N., Worthington-White, D., Garden, O. A. & Musk, P. Apoptosis of leukemic cells accompanies reduction in intracellular pH after targeted inhibition of the Na+/H+ exchanger. Blood 95, 1427–1434 (2000).
Harley, W. et al. Dual inhibition of sodium-mediated proton and calcium efflux triggers non-apoptotic cell death in malignant gliomas. Brain Res. 1363, 159–169 (2010).
Masereel, B., Pochet, L. & Laeckmann, D. An overview of inhibitors of Na+/H+ exchanger. Eur. J. Med. Chem. 38, 547–554 (2003).
Avkiran, M., Cook, A. R. & Cuello, F. Targeting Na+/H+ exchanger regulation for cardiac protection: a RSKy approach? Curr. Opin. Pharmacol. 8, 133–140 (2008).
Mentzer, R. M. Jr et al. Sodium-hydrogen exchange inhibition by cariporide to reduce the risk of ischemic cardiac events in patients undergoing coronary artery bypass grafting: results of the EXPEDITION study. Ann. Thorac Surg. 85, 1261–1270 (2008).
Counillon, L., Bouret, Y., Marchiq, I. & Pouyssegur, J. Na+/H+ antiporter (NHE1) and lactate/H+ symporters (MCTs) in pH homeostasis and cancer metabolism. Biochim. Biophys. Acta 1863, 2465–2480 (2016).
Amith, S. R., Wilkinson, J. M. & Fliegel, L. Na+/H+ exchanger NHE1 regulation modulates metastatic potential and epithelial-mesenchymal transition of triple-negative breast cancer cells. Oncotarget 7, 21091–21113 (2016).
Andersen, A. P. et al. Roles of acid-extruding ion transporters in regulation of breast cancer cell growth in a 3-dimensional microenvironment. Mol. Cancer 15, 45 (2016).
Meehan, J. et al. Inhibition of pH regulation as a therapeutic strategy in hypoxic human breast cancer cells. Oncotarget 8, 42857–42875 (2017).
McIntyre, A. et al. Disrupting hypoxia-induced bicarbonate transport acidifies tumor cells and suppresses tumor growth. Cancer Res. 76, 3744–3755 (2016).
Wong, P., Kleemann, H. W. & Tannock, I. F. Cytostatic potential of novel agents that inhibit the regulation of intracellular pH. Br. J. Cancer 87, 238–245 (2002).
Petzoldt, A. G., Gleixner, E. M., Fumagalli, A., Vaccari, T. & Simons, M. Elevated expression of the V-ATPase C subunit triggers JNK-dependent cell invasion and overgrowth in a Drosophila epithelium. Dis. Model. Mech. 6, 689–700 (2013).
Grillo-Hill, B. K., Choi, C., Jimenez-Vidal, M. & Barber, D. L. Increased H+ efflux is sufficient to induce dysplasia and necessary for viability with oncogene expression. eLife http://dx.doi.org/10.7554/eLife.03270 (2015).
Ibrahim Hashim, A. et al. Reduction of metastasis using a non-volatile buffer. Clin. Exp. Metastasis 28, 841–849 (2011).
Ibrahim-Hashim, A. et al. Systemic buffers inhibit carcinogenesis in TRAMP mice. J. Urol. 188, 624–631 (2012).
Ibrahim-Hashim, A. et al. Free base lysine increases survival and reduces metastasis in prostate cancer model. J. Cancer Sci. Ther. 4, (Suppl. 1) JCST-S1-004 (2011).
Silva, A. S., Yunes, J. A., Gillies, R. J. & Gatenby, R. A. The potential role of systemic buffers in reducing intratumoral extracellular pH and acid-mediated invasion. Cancer Res. 69, 2677–2684 (2009). This study shows that systemic bicarbonate administration may significantly reduce tumour growth and invasion in mice without altering the pH of blood or normal tissues.
Wojtkowiak, J. W., Verduzco, D., Schramm, K. J. & Gillies, R. J. Drug resistance and cellular adaptation to tumor acidic pH microenvironment. Mol. Pharm. 8, 2032–2038 (2011).
Chao, M. et al. A nonrandomized cohort and a randomized study of local control of large hepatocarcinoma by targeting intratumoral lactic acidosis. eLife 5, e15691 (2016).
Calcinotto, A. et al. Modulation of microenvironment acidity reverses anergy in human and murine tumor-infiltrating T lymphocytes. Cancer Res. 72, 2746–2756 (2012).
Pilon-Thomas, S. et al. Neutralization of tumor acidity improves antitumor responses to immunotherapy. Cancer Res. 76, 1381–1390 (2016).
Adeva-Andany, M. M., Fernandez-Fernandez, C., Mourino-Bayolo, D., Castro-Quintela, E. & Dominguez-Montero, A. Sodium bicarbonate therapy in patients with metabolic acidosis. ScientificWorldJournal 2014, 627673 (2014).
Danhier, F., Feron, O. & Preat, V. To exploit the tumor microenvironment: passive and active tumor targeting of nanocarriers for anti-cancer drug delivery. J. Control Release 148, 135–146 (2010).
He, X., Li, J., An, S. & Jiang, C. pH-sensitive drug-delivery systems for tumor targeting. Ther. Deliv. 4, 1499–1510 (2013).
Kanamala, M., Wilson, W. R., Yang, M., Palmer, B. D. & Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: a review. Biomaterials 85, 152–167 (2016).
Liu, J. et al. pH-sensitive nano-systems for drug delivery in cancer therapy. Biotechnol. Adv. 32, 693–710 (2014).
Subbarao, N. K. et al. pH-dependent bilayer destabilization by an amphipathic peptide. Biochemistry 26, 2964–2972 (1987).
Sakurai, Y. et al. Endosomal escape and the knockdown efficiency of liposomal-siRNA by the fusogenic peptide shGALA. Biomaterials 32, 5733–5742 (2011).
Miura, N., Akita, H., Tateshita, N., Nakamura, T. & Harashima, H. Modifying antigen-encapsulating liposomes with KALA facilitates MHC class I antigen presentation and enhances anti-tumor effects. Mol. Ther. 25, 1003–1013 (2017).
Miura, N., Shaheen, S. M., Akita, H., Nakamura, T. & Harashima, H. A KALA-modified lipid nanoparticle containing CpG-free plasmid DNA as a potential DNA vaccine carrier for antigen presentation and as an immune-stimulative adjuvant. Nucleic Acids Res. 43, 1317–1331 (2015).
Andreev, O. A. et al. Mechanism and uses of a membrane peptide that targets tumors and other acidic tissues in vivo. Proc. Natl Acad. Sci. USA 104, 7893–7898 (2007).
Reshetnyak, Y. K., Andreev, O. A., Lehnert, U. & Engelman, D. M. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc. Natl Acad. Sci. USA 103, 6460–6465 (2006).
Reshetnyak, Y. K., Andreev, O. A., Segala, M., Markin, V. S. & Engelman, D. M. Energetics of peptide (pHLIP) binding to and folding across a lipid bilayer membrane. Proc. Natl Acad. Sci. USA 105, 15340–15345 (2008). References 160–162 are a series of papers from the Engelman group reporting the development of pHLIP technology and its potential use in tumour imaging and drug delivery.
Onyango, J. O. et al. Noncanonical amino acids to improve the pH response of pHLIP insertion at tumor acidity. Angew. Chem. Int. Ed Engl. 54, 3658–3663 (2015).
Wijesinghe, D., Arachchige, M. C., Lu, A., Reshetnyak, Y. K. & Andreev, O. A. pH dependent transfer of nano-pores into membrane of cancer cells to induce apoptosis. Sci. Rep. 3, 3560 (2013).
Yao, L. et al. pHLIP peptide targets nanogold particles to tumors. Proc. Natl Acad. Sci. USA 110, 465–470 (2013).
Yao, L., Daniels, J., Wijesinghe, D., Andreev, O. A. & Reshetnyak, Y. K. pHLIP®-mediated delivery of PEGylated liposomes to cancer cells. J. Control Release 167, 228–237 (2013).
Srivastava, A. et al. Nanosomes carrying doxorubicin exhibit potent anticancer activity against human lung cancer cells. Sci. Rep. 6, 38541 (2016).
Hu, X. et al. Stimuli-responsive polymersomes for biomedical applications. Biomacromolecules 18, 649–673 (2017).
Simon-Gracia, L. et al. Paclitaxel-loaded polymersomes for enhanced intraperitoneal chemotherapy. Mol. Cancer Ther. 15, 670–679 (2016).
Pegoraro, C. et al. Enhanced drug delivery to melanoma cells using PMPC-PDPA polymersomes. Cancer Lett. 334, 328–337 (2013).
Colley, H. E. et al. Polymersome-mediated delivery of combination anticancer therapy to head and neck cancer cells: 2D and 3D in vitro evaluation. Mol. Pharm. 11, 1176–1188 (2014).
Spencer, J. A. et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature 508, 269–273 (2014).
Weiss, G. J. et al. Phase 1 study of the safety, tolerability, and pharmacokinetics of TH-302, a hypoxia-activated prodrug, in patients with advanced solid malignancies. Clin. Cancer Res. 17, 2997–3004 (2011).
Piermarini, P. M., Kim, E. Y. & Boron, W. F. Evidence against a direct interaction between intracellular carbonic anhydrase II and pure C-terminal domains of SLC4 bicarbonate transporters. J. Biol. Chem. 282, 1409–1421 (2007).
Al-Samir, S. et al. Activity and distribution of intracellular carbonic anhydrase II and their effects on the transport activity of anion exchanger AE1/SLC4A1. J. Physiol. 591, 4963–4982 (2013).
Hulikova, A., Aveyard, N., Harris, A. L., Vaughan-Jones, R. D. & Swietach, P. Intracellular carbonic anhydrase activity sensitizes cancer cell pH signaling to dynamic changes in CO2 partial pressure. J. Biol. Chem. 289, 25418–25430 (2014).
Noor, S. I. et al. Analysis of the binding moiety mediating the interaction between monocarboxylate transporters and carbonic anhydrase II. J. Biol. Chem. 290, 4476–4486 (2015).
Kolosenko, I., Avnet, S., Baldini, N., Viklund, J. & De Milito, A. Therapeutic implications of tumor interstitial acidification. Semin. Cancer Biol. 43, 119–133 (2017).
Chen, L. Q. & Pagel, M. D. Evaluating pH in the extracellular tumor microenvironment using CEST MRI and other imaging methods. Adv. Radiol. 2015, 206405 (2015).
Wyatt, L. C., Lewis, J. S., Andreev, O. A., Reshetnyak, Y. K. & Engelman, D. M. Applications of pHLIP technology for cancer imaging and therapy. Trends Biotechnol. 35, 653–366 (2017).
Chen, L. Q. et al. Evaluations of extracellular pH within in vivo tumors using acidoCEST MRI. Magn. Reson. Med. 72, 1408–1417 (2014).
Akhenblit, P. J. et al. Assessing metabolic changes in response to mTOR inhibition in a mantle cell lymphoma xenograft model using AcidoCEST MRI. Mol. Imaging 15, 1536012116645439 (2016).
Chen, L. Q. et al. Evaluations of tumor acidosis within in vivo tumor models using parametric maps generated with acido CEST MRI. Mol. Imag. Biol. 17, 488–496 (2015).
Jones, K. M. et al. Clinical translation of tumor acidosis measurements with AcidoCEST MRI. Mol. Imag. Biol. 19, 617–625 (2016).
Longo, D. L. et al. In vivo imaging of tumour metabolism and acidosis by combining PET and MRI-CEST pH imaging. Cancer Res. 76, 6463–6470 (2016).
Wykoff, C. C. et al. Hypoxia-inducible expression of tumor-associated carbonic anhydrases. Cancer Res. 60, 7075–7083 (2000).
Matthews, H., Ranson, M. & Kelso, M. J. Anti-tumour/metastasis effects of the potassium-sparing diuretic amiloride: an orally active anti-cancer drug waiting for its call-of-duty? Int. J. Cancer 129, 2051–2061 (2011).
Hosogi, S. et al. An inhibitor of Na+/H+ exchanger (NHE), ethyl-isopropyl amiloride (EIPA), diminishes proliferation of MKN28 human gastric cancer cells by decreasing the cytosolic Cl− concentration via DIDS-sensitive pathways. Cell Physiol. Biochem. 30, 1241–1253 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01791595 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00357682 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02518373 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01748500 (2017).
Hernandez, A., Serrano-Bueno, G., Perez-Castineira, J. R. & Serrano, A. Intracellular proton pumps as targets in chemotherapy: V-ATPases and cancer. Curr. Pharm. Des. 18, 1383–1394 (2012).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT00087022 (2014).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT01028755 (2015).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02215850 (2016).
US National Library of Medicine. ClinicalTrials.gov https://clinicaltrials.gov/ct2/show/NCT02216669 (2017).
Lock, F. E. et al. Targeting carbonic anhydrase IX depletes breast cancer stem cells within the hypoxic niche. Oncogene 32, 5210–5219 (2013).
Acknowledgements
This work was supported by grants from the Fonds National de la Recherche Scientifique (FRS-FNRS), the Télévie, the Belgian Foundation against Cancer, the J. Maisin Foundation and an Action de Recherche Concertée from the Fédération Wallonie-Bruxelles (ARC 14/19-058). C.C. is a senior FRS-FNRS postdoctoral fellow.
Author information
Authors and Affiliations
Contributions
O.F. and C.C. wrote the article and conceived the figures and other display items. O.F. and C.C. reviewed and edited the article before final submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Symporter
-
An integral membrane protein that mediates the unidirectional co-transport of two molecules across a cell membrane.
- Glutaminolysis
-
The metabolism of glutamine that yields a variety of products, including glutamate, α-ketoglutarate, malate, pyruvate and lactate.
- Syncytium
-
A group of cells coupled by gap junction-mediated connections.
- Metabolic symbiosis
-
A biological cooperation between two cell populations, one consuming a metabolite that is produced by the other, which in turn derives growth or survival benefits from this exchange.
- Mitochondrial fragmentation
-
A change in morphology of mitochondria from a highly branched network to a fragmented vesicular form.
- Acid proteolysis
-
Enzyme-catalysed protein breakdown promoted by an acidic environment.
- Preinvasive cancer cells
-
Neoplastic epithelial cells that in vivo have not breached the basement membrane, usually referred to as carcinoma in situ.
- T cell anergy
-
A tolerance mechanism in which a functionally inactive lymphocyte remains alive in a hyporesponsive state.
- Antacids
-
Substances that neutralize acidity, especially in the stomach.
- Zollinger–Ellison syndrome
-
A disease in which tumours in the pancreas or the upper part of the small intestine provoke an overproduction of acid into the stomach, resulting in peptic ulcers.
- Transarterial embolization
-
A surgical procedure aiming to block blood supply to a tumour or an abnormal area of tissue.
- Hypokalaemia
-
A deficiency of potassium in the blood serum.
- QT interval prolongation
-
An abnormally prolonged interval between the start of the Q wave and the end of the T wave in the electrical cycle of the heart, which may lead to cardiac arrest.
Rights and permissions
About this article
Cite this article
Corbet, C., Feron, O. Tumour acidosis: from the passenger to the driver's seat. Nat Rev Cancer 17, 577–593 (2017). https://doi.org/10.1038/nrc.2017.77
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2017.77
This article is cited by
-
Modulating ferroptosis sensitivity: environmental and cellular targets within the tumor microenvironment
Journal of Experimental & Clinical Cancer Research (2024)
-
The roles and molecular mechanisms of non-coding RNA in cancer metabolic reprogramming
Cancer Cell International (2024)
-
Deciphering the role of neddylation in tumor microenvironment modulation: common outcome of multiple signaling pathways
Biomarker Research (2024)
-
EphA2-specific microvesicles derived from tumor cells facilitate the targeted delivery of chemotherapeutic drugs for osteosarcoma therapy
Journal of Nanobiotechnology (2024)
-
Cancer immunometabolism: advent, challenges, and perspective
Molecular Cancer (2024)