Abstract
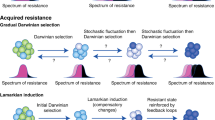
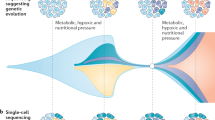
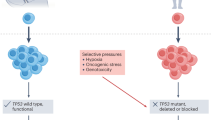
How can we stop cancer progression? Current strategies depend on modelling progression as the balanced outcome of mutations in, and expression of, tumour suppressor genes and oncogenes. New treatments emerge from successful attempts to tip that balance, but secondary mutational escape from those treatments has become a major impediment because it leads to resistance. In this Opinion article, we argue for a return to an earlier stratagem: tumour cell reversion. Treatments based on selection and analysis of stable revertants could create more durable remissions by reducing the selective pressure that leads to rapid drug resistance.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Zhao, B. et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21, 2747–2761 (2007).
Huang, S. & Ingber, D. E. The structural and mechanical complexity of cell-growth control. Nat. Cell Biol. 1, E131–E138 (1999).
Luria, S. E. & Delbruck, M. Mutations of bacteria from virus sensitivity to virus resistance. Genetics 28, 491–511 (1943).
Pollack, R. E., Green, H. & Todaro, G. J. Growth control in cultured cells: selection of sublines with increased sensitivity to contact inhibition and decreased tumor-producing ability. Proc. Natl Acad. Sci. USA 60, 126 (1968).
Steinberg, B., Pollack, R., Topp, W. & Botchan, M. Isolation and characterization of T antigen-negative revertants from a line of transformed rat cells containing one copy of the SV40 genome. Cell 13, 19–32 (1978).
Varmus, H. E., Quintrell, N. & Wyke, J. Revertants of an ASV-transformed rat cell line have lost the complete provius or sustained mutations in src. Virology 108, 28–46 (1981).
Pollack, R., Wolman, S. & Vogel, A. Reversion of virus-transformed cell lines: hyperploidy accompanies retention of viral genes. Nature 228, 938 (1970).
Shin, S.-I., Freedman, V. H., Risser, R. & Pollack, R. Tumorigenicity of virus-transformed cells in nude mice is correlated specifically with anchorage independent growth in vitro. Proc. Natl Acad. Sci. USA 72, 4435–4439 (1975).
Noda, M., Selinger, Z., Scolnick, E. M. & Bassin, R. H. Flat revertants isolated from Kirsten sarcoma virus-transformed cells are resistant to the action of specific oncogenes. Proc. Natl Acad. Sci. USA 80, 5602–5606 (1983).
Fujita, H. et al. A specific protein, p92, detected in flat revertants derived from NIH/3T3 transformed by human activated c-Ha-ras oncogene. Exp. Cell Res. 186, 115–121 (1990).
Noda, M. et al. Detection of genes with a potential for suppressing the transformed phenotype associated with activated ras genes. Proc. Natl Acad. Sci. USA 86, 162–166 (1989).
Weinberg, R. A. Tumor suppressor genes. Science 254, 1138–1146 (1991).
White, D. E. et al. Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction. Cancer Cell 6, 159–170 (2004).
Rogers, M. S. et al. Spontaneous reversion of the angiogenic phenotype to a nonangiogenic and dormant state in human tumors. Mol. Cancer Res. 12, 754–764 (2014).
Van Dyke, T. & Jacks, T. Cancer modeling in the modern era: progress and challenges. Cell 108, 135–144 (2002).
Telerman, A. & Amson, R. The molecular programme of tumour reversion: the steps beyond malignant transformation. Nat. Rev. Cancer 9, 206–216 (2009).
Bhat, A. A. et al. Claudin-7 expression induces mesenchymal to epithelial transformation (MET) to inhibit colon tumorigenesis. Oncogene 34, 4570–4580 (2015).
Lespagnol, A. et al. Exosome secretion, including the DNA damage-induced p53-dependent secretory pathway, is severely compromised in TSAP6/Steap3-null mice. Cell Death Differ. 15, 1723–1733 (2008).
Tuynder, M. et al. Biological models and genes of tumor reversion: cellular reprogramming through tpt1/TCTP and SIAH-1. Proc. Natl Acad. Sci. USA 99, 14976–14981 (2002).
Yu, X., Harris, S. L. & Levine, A. J. The regulation of exosome secretion: a novel function of the p53 protein. Cancer Res. 66, 4795–4801 (2006).
Fujita, K. et al. Positive feedback between p53 and TRF2 during telomere-damage signalling and cellular senescence. Nat. Cell Biol. 12, 1205–1212 (2010).
Roperch, J.-P. et al. Inhibition of presenilin 1 expression is promoted by p53 and p21WAF-1 and results in apoptosis and tumor suppression. Nat. Med. 4, 835–838 (1998).
Yang, X. et al. Notch activation induces apoptosis in neural progenitor cells through a p53-dependent pathway. Dev. Biol. 269, 81–94 (2004).
Susini, L. et al. Siah-1 binds and regulates the function of Numb. Proc. Natl Acad. Sci. USA 98, 15067–15072 (2001).
Amson, R. et al. Reciprocal repression between P53 and TCTP. Nat. Med. 18, 91–99 (2012).
Hsu, P. D., Lander, E. S. & Zhang, F. Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014).
McCormick, F. KRAS as a therapeutic target. Clin. Cancer Res. 21, 1797–1801 (2015).
Misale, S. et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature 486, 532–536 (2012).
Diaz Jr, L. A. et al. The molecular evolution of acquired resistance to targeted EGFR blockade in colorectal cancers. Nature 486, 537–540 (2012).
Soverini, S. et al. Resistance to dasatinib in Philadelphia-positive leukemia patients and the presence or the selection of mutations at residues 315 and 317 in the BCR-ABL kinase domain. Haematologica 92, 401–404 (2007).
Mintz, B. & Illmensee, K. Normal genetically mosaic mice produced from malignant teratocarcinoma cells. Proc. Natl Acad. Sci. USA 72, 3585–3589 (1975).
Augeron, C. & Laboisse, C. L. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 44, 3961–3969 (1984).
Ablain, J., Nasr, R., Bazarbachi, A. & de The, H. The drug-induced degradation of oncoproteins: an unexpected Achilles' heel of cancer cells? Cancer Discov. 1, 117–127 (2011).
Nardella, C., Clohessy, J. G., Alimonti, A. & Pandolfi, P. P. Pro-senescence therapy for cancer treatment. Nat. Rev. Cancer 11, 503–511 (2011).
Xue, W. et al. Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas. Nature 445, 656–660 (2007).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Leekha, S., Terrell, C. L. & Edson, R. S. General priniples of antimicrobial therapy. Mayo Clinic Proc. 86, 156–167 (2011).
Stent, G. S. Max Delbruck, 1906–1981. Genetics 101, 1–16 (1982).
Watson, J. D. Salvador, E. Luria (13 August 1912–6 February 1991). Proc. Am. Philos. Soc. 143, 681–683 (1999).
Werner, R. Nature of DNA precursors. Nature 233, 99–103 (1971).
De Lucia, P. & Cairns, J. Isolation of an E. coli strain with a mutation affecting DNA polymerase. Nature 224, 1164–1166 (1969).
Cairns, J. The bacterial chromosome and its manner of replication as seen by autoradiography. J. Mol. Biol. 6, 208–213 (1963).
Potten, C. S., Hume, W. J., Reid, P. & Cairns, J. The segregation of DNA in epithelial stem cells. Cell 15, 899–906 (1978).
Cairns, J. & Foster, P. L. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128, 695–701 (1991).
Maisnier-Patin, S. & Roth, J. R. The origin of mutants under selection: how natural selection mimics mutagenesis (adaptive mutation). Cold Spring Harb. Perspect. Biol. 7, a018176 (2015).
Vogel, A. & Pollack, R. Isolation and characterization of revertant cell lines. IV. Direct selection of serum-revertant sublines of SV40-transformed 3T3 mouse cells. J. Cell. Physiol. 82, 189–198 (1973).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
FURTHER INFORMATION
PowerPoint slides
Rights and permissions
About this article
Cite this article
Powers, S., Pollack, R. Inducing stable reversion to achieve cancer control. Nat Rev Cancer 16, 266–270 (2016). https://doi.org/10.1038/nrc.2016.12
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.12
This article is cited by
-
Critical transition and reversion of tumorigenesis
Experimental & Molecular Medicine (2023)
-
Exosome-mediated delivery of SCD-1 siRNA promoted the death of anaplastic thyroid carcinoma cells via regulating ROS level
Clinical and Translational Oncology (2022)
-
RETRACTED ARTICLE: Identification of protein kinase inhibitors to reprogram breast cancer cells
Cell Death & Disease (2018)
-
A spotlight on bacterial mutations for 75 years
Nature (2018)
-
Attractor landscape analysis of colorectal tumorigenesis and its reversion
BMC Systems Biology (2016)