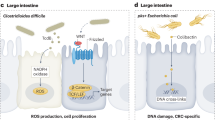
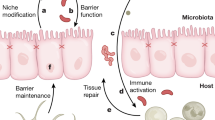
Abstract
Microbiota and host form a complex 'super-organism' in which symbiotic relationships confer benefits to the host in many key aspects of life. However, defects in the regulatory circuits of the host that control bacterial sensing and homeostasis, or alterations of the microbiome, through environmental changes (infection, diet or lifestyle), may disturb this symbiotic relationship and promote disease. Increasing evidence indicates a key role for the bacterial microbiota in carcinogenesis. In this Opinion article, we discuss links between the bacterial microbiota and cancer, with a particular focus on immune responses, dysbiosis, genotoxicity, metabolism and strategies to target the microbiome for cancer prevention.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Koch, R. in Tenth International Congress of Medicine 1 (August Hirschwald, Berlin, 1891).
Balkwill, F. & Mantovani, A. Inflammation and cancer: back to Virchow? Lancet 357, 539–545 (2001).
Trinchieri, G. Cancer and inflammation: an old intuition with rapidly evolving new concepts. Annu. Rev. Immunol. 30, 677–706 (2012).
Moore, P. S. & Chang, Y. Why do viruses cause cancer? Highlights of the first century of human tumour virology. Nature Rev. Cancer 10, 878–889 (2010).
Virchow, R. in Die krankhaften Geschwülste (ed. Virchow, R.) 57–101 (Verlag von August von Hirschwald, Berlin, 1863).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Turnbaugh, P. J. et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444, 1027–1031 (2006).
Smith, M. I. et al. Gut microbiomes of Malawian twin pairs discordant for kwashiorkor. Science 339, 548–554 (2013).
O'Hara, A. M. & Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 7, 688–693 (2006).
Savage, D. C. Microbial ecology of the gastrointestinal tract. Annu. Rev. Microbiol. 31, 107–133 (1977).
Gill, S. R. et al. Metagenomic analysis of the human distal gut microbiome. Science 312, 1355–1359 (2006).
Kau, A. L., Ahern, P. P., Griffin, N. W., Goodman, A. L. & Gordon, J. I. Human nutrition, the gut microbiome and the immune system. Nature 474, 327–336 (2011).
Fraher, M. H., O'Toole, P. W. & Quigley, E. M. Techniques used to characterize the gut microbiota: a guide for the clinician. Nature Rev. Gastroenterol. Hepatol 9, 312–322 (2012).
Consortium, H. M. P. Structure, function and diversity of the healthy human microbiome. Nature 486, 207–214 (2012).
Colditz, G. A., Sellers, T. A. & Trapido, E. Epidemiology — identifying the causes and preventability of cancer? Nature Rev. Cancer 6, 75–83 (2006).
Peto, J. Cancer epidemiology in the last century and the next decade. Nature 411, 390–395 (2001).
Grice, E. A. & Segre, J. A. The skin microbiome. Nature Rev. Microbiol. 9, 244–253 (2011).
Eckburg, P. B. et al. Diversity of the human intestinal microbial flora. Science 308, 1635–1638 (2005).
Wiest, R. & Garcia-Tsao, G. Bacterial translocation (BT) in cirrhosis. Hepatology 41, 422–433 (2005).
Seki, E. et al. TLR4 enhances TGF-β signaling and hepatic fibrosis. Nature Med. 13, 1324–1332 (2007).
Dapito, D. H. et al. Promotion of hepatocellular carcinoma by the intestinal microbiota and TLR4. Cancer Cell 21, 504–516 (2012).
Yoshimoto, S. et al. Obesity-induced gut microbial metabolite promotes liver cancer through senescence secretome. Nature 499, 97–101 (2013).
Sacksteder, M. R. Occurrence of spontaneous tumors in the germfree F344 rat. J. Natl Cancer Inst. 57, 1371–1373 (1976).
Schreiber, H., Nettesheim, P., Lijinsky, W., Richter, C. B. & Walburg, H. E. Jr Induction of lung cancer in germfree, specific-pathogen-free, and infected rats by N-nitrosoheptamethyleneimine: enhancement by respiratory infection. J. Natl Cancer Inst. 49, 1107–1114 (1972).
Reddy, B. S. et al. Colon carcinogenesis with azoxymethane and dimethylhydrazine in germ-free rats. Cancer Res. 35, 287–290 (1975).
Reddy, B. S. & Watanabe, K. Effect of intestinal microflora on 2,2′-dimethyl-4-aminobiphenyl-induced carcinogenesis in F344 rats. J. Natl Cancer Inst. 61, 1269–1271 (1978).
Reddy, B. S., Weisburger, J. H., Narisawa, T. & Wynder, E. L. Colon carcinogenesis in germ-free rats with 1,2-dimethylhydrazine and N-methyl-N′-nitro-N-nitrosoguanidine. Cancer Res. 34, 2368–2372 (1974).
Laqueur, G. L., Matsumoto, H. & Yamamoto, R. S. Comparison of the carcinogenicity of methylazoxymethanol-β-D-glucosiduronic acid in conventional and germfree Sprague-Dawley rats. J. Natl Cancer Inst. 67, 1053–1055 (1981).
Uronis, J. M. et al. Modulation of the intestinal microbiota alters colitis-associated colorectal cancer susceptibility. PLoS ONE 4, e6026 (2009).
Lofgren, J. L. et al. Lack of commensal flora in Helicobacter pylori-infected INS-GAS mice reduces gastritis and delays intraepithelial neoplasia. Gastroenterology 140, 210–220 (2011).
Li, Y. et al. Gut microbiota accelerate tumor growth via c-jun and STAT3 phosphorylation in APCMin/+ mice. Carcinogenesis 33, 1231–1238 (2012).
Vannucci, L. et al. Colorectal carcinogenesis in germ-free and conventionally reared rats: different intestinal environments affect the systemic immunity. Int. J. Oncol. 32, 609–617 (2008).
Dove, W. F. et al. Intestinal neoplasia in the ApcMin mouse: independence from the microbial and natural killer (beige locus) status. Cancer Res. 57, 812–814 (1997).
Chen, G. Y., Shaw, M. H., Redondo, G. & Nunez, G. The innate immune receptor Nod1 protects the intestine from inflammation-induced tumorigenesis. Cancer Res. 68, 10060–10067 (2008).
Yu, L. X. et al. Endotoxin accumulation prevents carcinogen-induced apoptosis and promotes liver tumorigenesis in rodents. Hepatology 52, 1322–1333 (2010).
Grivennikov, S. I. et al. Adenoma-linked barrier defects and microbial products drive IL-23/IL-17-mediated tumour growth. Nature 491, 254–258 (2012).
Klimesova, K. et al. Altered gut microbiota promotes colitis-associated cancer in IL-1 receptor-associated kinase M-deficient mice. Inflamm. Bowel Dis. 19, 1266–1277 (2013).
Lee, C. W. et al. Helicobacter pylori eradication prevents progression of gastric cancer in hypergastrinemic INS-GAS mice. Cancer Res. 68, 3540–3548 (2008).
Ma, J. L. et al. Fifteen-year effects of Helicobacter pylori, garlic, and vitamin treatments on gastric cancer incidence and mortality. J. Natl Cancer Inst. 104, 488–492 (2012).
Wong, B. C. et al. Helicobacter pylori eradication to prevent gastric cancer in a high-risk region of China: a randomized controlled trial. JAMA 291, 187–194 (2004).
Coley, W. B. Treatment of inoperable malignant tumors with the toxins of erysipelas and the Bacillus prodigiosus. Trans. Amer. Surg. Assn 12, 183–212 (1894).
Starnes, C. O. Coley's toxins in perspective. Nature 357, 11–12 (1992).
Shear, M. J. & Andervont, H. B. Chemical treatment of tumors. III. separation of hemorrhage-producing fraction of B. coli filtrate. Exp. Biol. Med. (Maywood) 34, 323–324 (1936).
Pradere, J. P., Dapito, D. H. & Schwabe, R. F. The yin and yang of toll-like receptors in cancer. Oncogene http://dx.doi.org/10.1038/onc.2013.302 (2013).
Garaude, J., Kent, A., van Rooijen, N. & Blander, J. M. Simultaneous targeting of Toll- and NOD-like receptors induces effective tumor-specific immune responses. Sci. Transl. Med. 4, 120ra16 (2012).
Peek, R. M. Jr & Blaser, M. J. Helicobacter pylori and gastrointestinal tract adenocarcinomas. Nature Rev. Cancer 2, 28–37 (2002).
Fox, J. G. & Wang, T. C. Inflammation, atrophy, and gastric cancer. J. Clin. Invest. 117, 60–69 (2007).
Islami, F. & Kamangar, F. Helicobacter pylori and esophageal cancer risk: a meta-analysis. Cancer Prev. Res. (Phila) 1, 329–338 (2008).
Caygill, C. P., Hill, M. J., Braddick, M. & Sharp, J. C. Cancer mortality in chronic typhoid and paratyphoid carriers. Lancet 343, 83–84 (1994).
Welton, J. C., Marr, J. S. & Friedman, S. M. Association between hepatobiliary cancer and typhoid carrier state. Lancet 1, 791–794 (1979).
Wotherspoon, A. C. et al. Regression of primary low-grade B-cell gastric lymphoma of mucosa-associated lymphoid tissue type after eradication of Helicobacter pylori. Lancet 342, 575–577 (1993).
Lecuit, M. et al. Immunoproliferative small intestinal disease associated with Campylobacter jejuni. N. Engl. J. Med. 350, 239–248 (2004).
Senff, N. J. et al. European Organization for Research and Treatment of Cancer and International Society for Cutaneous Lymphoma consensus recommendations for the management of cutaneous B-cell lymphomas. Blood 112, 1600–1609 (2008).
Ferreri, A. J. et al. Chlamydophila psittaci eradication with doxycycline as first-line targeted therapy for ocular adnexae lymphoma: final results of an international phase II trial. J. Clin. Oncol. 30, 2988–2994 (2012).
Grivennikov, S. I. Inflammation and colorectal cancer: colitis-associated neoplasia. Semin. Immunopathol. 35, 229–244 (2012).
Ochi, A. et al. MyD88 inhibition amplifies dendritic cell capacity to promote pancreatic carcinogenesis via Th2 cells. J. Exp. Med. 209, 1671–1687 (2012).
Michaud, D. S., Joshipura, K., Giovannucci, E. & Fuchs, C. S. A prospective study of periodontal disease and pancreatic cancer in US male health professionals. J. Natl Cancer Inst. 99, 171–175 (2007).
Farrell, J. J. et al. Variations of oral microbiota are associated with pancreatic diseases including pancreatic cancer. Gut 61, 582–588 (2012).
Pragman, A. A., Kim, H. B., Reilly, C. S., Wendt, C. & Isaacson, R. E. The lung microbiome in moderate and severe chronic obstructive pulmonary disease. PLoS ONE 7, e47305 (2012).
Sethi, S. & Murphy, T. F. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N. Engl. J. Med. 359, 2355–2365 (2008).
Houghton, A. M. Mechanistic links between COPD and lung cancer. Nature Rev. Cancer 13, 233–245 (2013).
Melkamu, T., Qian, X., Upadhyaya, P., O'Sullivan, M. G. & Kassie, F. Lipopolysaccharide enhances mouse lung tumorigenesis: a model for inflammation- driven lung cancer. Vet. Pathol. 50, 895–902 (2013).
Swann, J. B. et al. Demonstration of inflammation-induced cancer and cancer immunoediting during primary tumorigenesis. Proc. Natl Acad. Sci. USA 105, 652–656 (2008).
Mittal, D. et al. TLR4-mediated skin carcinogenesis is dependent on immune and radioresistant cells. EMBO J. 29, 2242–2252 (2010).
Cataisson, C. et al. IL-1R–MyD88 signaling in keratinocyte transformation and carcinogenesis. J. Exp. Med. 209, 1689–1702 (2012).
Backhed, F., Ley, R. E., Sonnenburg, J. L., Peterson, D. A. & Gordon, J. I. Host–bacterial mutualism in the human intestine. Science 307, 1915–1920 (2005).
Dethlefsen, L., McFall-Ngai, M. & Relman, D. A. An ecological and evolutionary perspective on human-microbe mutualism and disease. Nature 449, 811–818 (2007).
Eloe-Fadrosh, E. A. & Rasko, D. A. The human microbiome: from symbiosis to pathogenesis. Annu. Rev. Med. 64, 145–163 (2013).
Salzman, N. H., Underwood, M. A. & Bevins, C. L. Paneth cells, defensins, and the commensal microbiota: a hypothesis on intimate interplay at the intestinal mucosa. Semin. Immunol. 19, 70–83 (2007).
Nestle, F. O., Di Meglio, P., Qin, J. Z. & Nickoloff, B. J. Skin immune sentinels in health and disease. Nature Rev. Immunol. 9, 679–691 (2009).
Littman, D. R. & Rudensky, A. Y. Th17 and regulatory T cells in mediating and restraining inflammation. Cell 140, 845–858 (2010).
Pabst, O. New concepts in the generation and functions of IgA. Nature Rev. Immunol. 12, 821–832 (2012).
Ashida, H., Ogawa, M., Kim, M., Mimuro, H. & Sasakawa, C. Bacteria and host interactions in the gut epithelial barrier. Nature Chem. Biol. 8, 36–45 (2011).
van Nood, E. et al. Duodenal infusion of donor feces for recurrent Clostridium difficile. N. Engl. J. Med. 368, 407–415 (2013).
Kamada, N. et al. Regulated virulence controls the ability of a pathogen to compete with the gut microbiota. Science 336, 1325–1329 (2012).
Cornforth, D. M. & Foster, K. R. Competition sensing: the social side of bacterial stress responses. Nature Rev. Microbiol. 11, 285–293 (2013).
Arthur, J. C. et al. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338, 120–123 (2012).
Couturier-Maillard, A. et al. NOD2-mediated dysbiosis predisposes mice to transmissible colitis and colorectal cancer. J. Clin. Invest. 123, 700–711 (2013).
Hu, B. et al. Microbiota-induced activation of epithelial IL-6 signaling links inflammasome-driven inflammation with transmissible cancer. Proc. Natl Acad. Sci. USA 110, 9862–9867 (2013).
Khor, B., Gardet, A. & Xavier, R. J. Genetics and pathogenesis of inflammatory bowel disease. Nature 474, 307–317 (2011).
Houlston, R. S. et al. Meta-analysis of three genome-wide association studies identifies susceptibility loci for colorectal cancer at 1q41, 3q26.2, 12q13.13 and 20q13.33. Nature Genet. 42, 973–977 (2010).
Peters, U. et al. Identification of genetic susceptibility loci for colorectal tumors in a genome-wide meta-analysis. Gastroenterology 144, 799–807 (2013).
Velcich, A. et al. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science 295, 1726–1729 (2002).
Irvine, A. D., McLean, W. H. & Leung, D. Y. Filaggrin mutations associated with skin and allergic diseases. N. Engl. J. Med. 365, 1315–1327 (2011).
Costello, E. K. et al. Bacterial community variation in human body habitats across space and time. Science 326, 1694–1697 (2009).
Caporaso, J. G. et al. Moving pictures of the human microbiome. Genome Biol. 12, R50 (2011).
Holmes, E., Li, J. V., Marchesi, J. R. & Nicholson, J. K. Gut microbiota composition and activity in relation to host metabolic phenotype and disease risk. Cell. Metab. 16, 559–564 (2012).
Ley, R. E., Turnbaugh, P. J., Klein, S. & Gordon, J. I. Microbial ecology: human gut microbes associated with obesity. Nature 444, 1022–1023 (2006).
Ward, J. M. et al. Chronic active hepatitis and associated liver tumors in mice caused by a persistent bacterial infection with a novel Helicobacter species. J. Natl Cancer Inst. 86, 1222–1227 (1994).
Erdman, S. E. et al. Nitric oxide and TNF-α trigger colonic inflammation and carcinogenesis in Helicobacter hepaticus-infected, Rag2-deficient mice. Proc. Natl Acad. Sci. USA 106, 1027–1032 (2009).
Kim, S. C. et al. Variable phenotypes of enterocolitis in interleukin 10-deficient mice monoassociated with two different commensal bacteria. Gastroenterology 128, 891–906 (2005).
Wu, S. et al. A human colonic commensal promotes colon tumorigenesis via activation of T helper type 17 T cell responses. Nature Med. 15, 1016–1022 (2009).
Boulard, O., Kirchberger, S., Royston, D. J., Maloy, K. J. & Powrie, F. M. Identification of a genetic locus controlling bacteria-driven colitis and associated cancer through effects on innate inflammation. J. Exp. Med. 209, 1309–1324 (2012).
Rao, V. P. et al. Proinflammatory CD4+ CD45RBhi lymphocytes promote mammary and intestinal carcinogenesis in ApcMin/+ mice. Cancer Res. 66, 57–61 (2006).
Garrett, W. S. et al. Colitis-associated colorectal cancer driven by T-bet deficiency in dendritic cells. Cancer Cell 16, 208–219 (2009).
Zhang, H. L. et al. Profound impact of gut homeostasis on chemically-induced pro-tumorigenic inflammation and hepatocarcinogenesis in rats. J. Hepatol 57, 803–812 (2012).
Ley, R. E. et al. Obesity alters gut microbial ecology. Proc. Natl Acad. Sci. USA 102, 11070–11075 (2005).
Cotillard, A. et al. Dietary intervention impact on gut microbial gene richness. Nature 500, 585–588 (2013).
Le Chatelier, E. et al. Richness of human gut microbiome correlates with metabolic markers. Nature 500, 541–546 (2013).
Calle, E. E. & Kaaks, R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nature Rev. Cancer 4, 579–591 (2004).
Devkota, S. et al. Dietary-fat-induced taurocholic acid promotes pathobiont expansion and colitis in Il10−/− mice. Nature 487, 104–108 (2012).
Chen, W., Liu, F., Ling, Z., Tong, X. & Xiang, C. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS ONE 7, e39743 (2012).
Sanapareddy, N. et al. Increased rectal microbial richness is associated with the presence of colorectal adenomas in humans. ISME J. 6, 1858–1868 (2012).
Sobhani, I. et al. Microbial dysbiosis in colorectal cancer (CRC) patients. PLoS ONE 6, e16393 (2011).
Wang, T. et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers. ISME J. 6, 320–329 (2011).
Kostic, A. D. et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 22, 292–298 (2012).
Kostic, A. D. et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe 14, 207–215 (2013).
McCoy, A. N. et al. Fusobacterium is associated with colorectal adenomas. PLoS ONE 8, e53653 (2013).
Rubinstein, M. R. et al. Fusobacterium nucleatum promotes colorectal carcinogenesis by modulating E-cadherin/β-catenin signaling via its FadA adhesin. Cell Host Microbe 14, 195–206 (2013).
Allen-Vercoe, E., Strauss, J. & Chadee, K. Fusobacterium nucleatum: an emerging gut pathogen? Gut Microbes 2, 294–298 (2011).
Stecher, B. et al. Gut inflammation can boost horizontal gene transfer between pathogenic and commensal Enterobacteriaceae. Proc. Natl Acad. Sci. USA 109, 1269–1274 (2012).
Elinav, E., Strowig, T., Henao-Mejia, J. & Flavell, R. A. Regulation of the antimicrobial response by NLR proteins. Immunity 34, 665–679 (2011).
Winter, S. E. et al. Host-derived nitrate boosts growth of E. coli in the inflamed gut. Science 339, 708–711 (2013).
Lupp, C. et al. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2, 204 (2007).
Morgan, X. C. et al. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol. 13, R79 (2012).
Vijay-Kumar, M. et al. Metabolic syndrome and altered gut microbiota in mice lacking Toll-like receptor 5. Science 328, 228–231 (2012).
Patwa, L. G. et al. Chronic intestinal inflammation induces stress-response genes in commensal Escherichia coli. Gastroenterology 141, 1842–1851 (2011).
Hajishengallis, G., Darveau, R. P. & Curtis, M. A. The keystone-pathogen hypothesis. Nature Rev. Microbiol. 10, 717–725 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Moresco, E. M., LaVine, D. & Beutler, B. Toll-like receptors. Curr. Biol. 21, R488–R493 (2011).
Fukata, M. et al. Toll-like receptor-4 promotes the development of colitis-associated colorectal tumors. Gastroenterology 133, 1869–1881 (2007).
Fukata, M. et al. Constitutive activation of epithelial TLR4 augments inflammatory responses to mucosal injury and drives colitis-associated tumorigenesis. Inflamm Bowel Dis. 17, 1464–1473 (2011).
Tye, H. et al. STAT3-driven upregulation of TLR2 promotes gastric tumorigenesis independent of tumor inflammation. Cancer Cell 22, 466–478 (2012).
Ngo, V. N. et al. Oncogenically active MYD88 mutations in human lymphoma. Nature 470, 115–119 (2011).
Brandl, K. et al. MyD88 signaling in nonhematopoietic cells protects mice against induced colitis by regulating specific EGF receptor ligands. Proc. Natl Acad. Sci. USA 107, 19967–19972 (2010).
Neufert, C. et al. Tumor fibroblast–derived epiregulin promotes growth of colitis-associated neoplasms through ERK. J. Clin. Invest. 123, 1428–1443 (2013).
Naugler, W. E. et al. Gender disparity in liver cancer due to sex differences in MyD88-dependent IL-6 production. Science 317, 121–124 (2007).
Rakoff-Nahoum, S. & Medzhitov, R. Regulation of spontaneous intestinal tumorigenesis through the adaptor protein MyD88. Science 317, 124–127 (2007).
Lee, S. H. et al. ERK activation drives intestinal tumorigenesis in Apcmin/+ mice. Nature Med. 16, 665–670 (2010).
Kennedy, C. L. et al. Differential role of MyD88 and Mal/TIRAP in TLR2-mediated gastric tumourigenesis. Oncogene http://dx.doi.org/10.1038/onc.2013.205 (2013).
Salcedo, R. et al. MyD88-mediated signaling prevents development of adenocarcinomas of the colon: role of interleukin 18. J. Exp. Med. 207, 1625–1636 (2010).
Cho, J. H. The genetics and immunopathogenesis of inflammatory bowel disease. Nature Rev. Immunol. 8, 458–466 (2008).
Kobayashi, K. S. et al. Nod2-dependent regulation of innate and adaptive immunity in the intestinal tract. Science 307, 731–734 (2005).
Petnicki-Ocwieja, T. et al. Nod2 is required for the regulation of commensal microbiota in the intestine. Proc. Natl Acad. Sci. USA 106, 15813–15818 (2009).
Rehman, A. et al. Nod2 is essential for temporal development of intestinal microbial communities. Gut 60, 1354–1362 (2011).
McGovern, D. P. et al. Association between a complex insertion/deletion polymorphism in NOD1 (CARD4) and susceptibility to inflammatory bowel disease. Hum. Mol. Genet. 14, 1245–1250 (2005).
Allen, I. C. et al. The NLRP3 inflammasome functions as a negative regulator of tumorigenesis during colitis-associated cancer. J. Exp. Med. 207, 1045–1056 (2010).
Allen, I. C. et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-κB signaling. Immunity 36, 742–754 (2012).
Hu, B. et al. Inflammation-induced tumorigenesis in the colon is regulated by caspase-1 and NLRC4. Proc. Natl Acad. Sci. USA 107, 21635–21640 (2010).
Travaglione, S., Fabbri, A. & Fiorentini, C. The Rho-activating CNF1 toxin from pathogenic E. coli: a risk factor for human cancer development? Infect. Agent Cancer 3, 4 (2008).
Nesic, D., Hsu, Y. & Stebbins, C. E. Assembly and function of a bacterial genotoxin. Nature 429, 429–433 (2004).
Cuevas-Ramos, G. et al. Escherichia coli induces DNA damage in vivo and triggers genomic instability in mammalian cells. Proc. Natl Acad. Sci. USA 107, 11537–11542 (2010).
Smith, J. L. & Bayles, D. O. The contribution of cytolethal distending toxin to bacterial pathogenesis. Crit. Rev. Microbiol. 32, 227–248 (2006).
Elwell, C. A. & Dreyfus, L. A. DNase I homologous residues in CdtB are critical for cytolethal distending toxin-mediated cell cycle arrest. Mol. Microbiol. 37, 952–963 (2000).
Fox, J. G. et al. Gastroenteritis in NF-κB-deficient mice is produced with wild-type Camplyobacter jejuni but not with C. jejuni lacking cytolethal distending toxin despite persistent colonization with both strains. Infect. Immun. 72, 1116–1125 (2004).
Shen, Z. et al. Cytolethal distending toxin promotes Helicobacter cinaedi-associated typhlocolitis in interleukin-10-deficient mice. Infect. Immun. 77, 2508–2516 (2009).
Nougayrede, J. P. et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851 (2006).
Buc, E. et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLoS ONE 8, e56964 (2013).
Garrett, W. S. et al. Enterobacteriaceae act in concert with the gut microbiota to induce spontaneous and maternally transmitted colitis. Cell Host Microbe 8, 292–300 (2010).
Putze, J. et al. Genetic structure and distribution of the colibactin genomic island among members of the family Enterobacteriaceae. Infect. Immun. 77, 4696–4703 (2009).
Carbonero, F., Benefiel, A. C., Alizadeh-Ghamsari, A. H. & Gaskins, H. R. Microbial pathways in colonic sulfur metabolism and links with health and disease. Front. Physiol. 3, 448 (2012).
Huycke, M. M. & Gaskins, H. R. Commensal bacteria, redox stress, and colorectal cancer: mechanisms and models. Exp. Biol. Med. (Maywood) 229, 586–597 (2004).
Wang, X. & Huycke, M. M. Extracellular superoxide production by Enterococcus faecalis promotes chromosomal instability in mammalian cells. Gastroenterology 132, 551–561 (2007).
Wang, X. et al. 4-hydroxy-2-nonenal mediates genotoxicity and bystander effects caused by Enterococcus faecalis-infected macrophages. Gastroenterology 142, 543–551 (2012).
Balish, E. & Warner, T. Enterococcus faecalis induces inflammatory bowel disease in interleukin-10 knockout mice. Am. J. Pathol. 160, 2253–2257 (2002).
Castellarin, M. et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res. 22, 299–306 (2012).
Attene-Ramos, M. S., Wagner, E. D., Plewa, M. J. & Gaskins, H. R. Evidence that hydrogen sulfide is a genotoxic agent. Mol. Cancer Res. 4, 9–14 (2006).
Ohnishi, N. et al. Transgenic expression of Helicobacter pylori CagA induces gastrointestinal and hematopoietic neoplasms in mouse. Proc. Natl Acad. Sci. USA 105, 1003–1008 (2008).
Han, Y. W. et al. Identification and characterization of a novel adhesin unique to oral fusobacteria. J. Bacteriol. 187, 5330–5340 (2005).
Philipp, B. Bacterial degradation of bile salts. Appl. Microbiol. Biotechnol. 89, 903–915 (2011).
Bernstein, C. et al. Carcinogenicity of deoxycholate, a secondary bile acid. Arch. Toxicol. 85, 863–871 (2011).
Quante, M. et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell 21, 36–51 (2012).
Nyangale, E. P., Mottram, D. S. & Gibson, G. R. Gut microbial activity, implications for health and disease: the potential role of metabolite analysis. J. Proteome Res. 11, 5573–5585 (2012).
Windey, K., De Preter, V. & Verbeke, K. Relevance of protein fermentation to gut health. Mol. Nutr. Food Res. 56, 184–196 (2012).
Hawksworth, G. M. & Hill, M. J. Bacteria and the N-nitrosation of secondary amines. Br. J. Cancer 25, 520–526 (1971).
Alam, B. S., Saporoschetz, I. B. & Epstein, S. S. Synthesis of nitrosopiperidine from nitrate and piperidine in the gastro-intestinal tract of the rat. Nature 232, 199–200 (1971).
Russell, W. R. et al. High-protein, reduced-carbohydrate weight-loss diets promote metabolite profiles likely to be detrimental to colonic health. Am. J. Clin. Nutr. 93, 1062–1072 (2011).
Bindels, L. B. et al. Gut microbiota-derived propionate reduces cancer cell proliferation in the liver. Br. J. Cancer 107, 1337–1344 (2012).
Donohoe, D. R. et al. The microbiome and butyrate regulate energy metabolism and autophagy in the mammalian colon. Cell. Metab. 13, 517–526 (2011).
Hu, S. et al. The microbe-derived short chain fatty acid butyrate targets miRNA-dependent p21 gene expression in human colon cancer. PLoS ONE 6, e16221 (2011).
Maslowski, K. M. et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461, 1282–1286 (2009).
Eberhardt, M. V., Lee, C. Y. & Liu, R. H. Antioxidant activity of fresh apples. Nature 405, 903–904 (2000).
DeWeerdt, S. Food: The omnivore's labyrinth. Nature 471, S22–S24 (2011).
Yang, C. S., Wang, X., Lu, G. & Picinich, S. C. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nature Rev. Cancer 9, 429–439 (2009).
van Duynhoven, J. et al. Metabolic fate of polyphenols in the human superorganism. Proc. Natl Acad. Sci. USA 108 (Suppl. 1), 4531–4538 (2011).
Fardet, A. et al. Metabolomics provide new insight on the metabolism of dietary phytochemicals in rats. J. Nutr. 138, 1282–1287 (2008).
Dutton, R. J. & Turnbaugh, P. J. Taking a metagenomic view of human nutrition. Curr. Opin. Clin. Nutr. Metab. Care 15, 448–454 (2012).
Chang, M. C. & Keasling, J. D. Production of isoprenoid pharmaceuticals by engineered microbes. Nature Chem. Biol. 2, 674–681 (2006).
Mabrok, H. B. et al. Lignan transformation by gut bacteria lowers tumor burden in a gnotobiotic rat model of breast cancer. Carcinogenesis 33, 203–208 (2012).
Adlercreutz, H. Phyto-oestrogens and cancer. Lancet Oncol. 3, 364–373 (2002).
Haiser, H. J. & Turnbaugh, P. J. Is it time for a metagenomic basis of therapeutics? Science 336, 1253–1255 (2012).
Wallace, B. D. et al. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science 330, 831–835 (2010).
Kassie, F. et al. Intestinal microflora plays a crucial role in the genotoxicity of the cooked food mutagen 2-amino-3-methylimidazo [4,5-f]quinoline. Carcinogenesis 22, 1721–1725 (2001).
Hirayama, K. et al. Effects of human intestinal flora on mutagenicity of and DNA adduct formation from food and environmental mutagens. Carcinogenesis 21, 2105–2111 (2000).
Vanhaecke, L. et al. Intestinal bacteria metabolize the dietary carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine following consumption of a single cooked chicken meal in humans. Food Chem. Toxicol. 46, 140–148 (2008).
Sharp, J. O., Wood, T. K. & Alvarez-Cohen, L. Aerobic biodegradation of N-nitrosodimethylamine (NDMA) by axenic bacterial strains. Biotechnol. Bioeng. 89, 608–618 (2005).
Seitz, H. K. & Stickel, F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nature Rev. Cancer 7, 599–612 (2007).
Seitz, H. K. et al. Possible role of acetaldehyde in ethanol-related rectal cocarcinogenesis in the rat. Gastroenterology 98, 406–413 (1990).
Plottel, C. S. & Blaser, M. J. Microbiome and malignancy. Cell Host Microbe 10, 324–335 (2011).
Markle, J. G. et al. Sex differences in the gut microbiome drive hormone-dependent regulation of autoimmunity. Science 339, 1084–1088 (2013).
Chung, H. et al. Gut immune maturation depends on colonization with a host-specific microbiota. Cell 149, 1578–1593 (2012).
Singh, S., Eldin, C., Kowalczewska, M. & Raoult, D. Axenic culture of fastidious and intracellular bacteria. Trends Microbiol. 21, 92–99 (2013).
Bull, A. T. The renaissance of continuous culture in the post-genomics age. J. Ind. Microbiol. Biotechnol. 37, 993–1021 (2010).
Arthur, J. C. & Jobin, C. The complex interplay between inflammation, the microbiota and colorectal cancer. Gut Microbes 4, 253–258 (2013).
Hooper, L. V., Littman, D. R. & Macpherson, A. J. Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012).
Ubeda, C. et al. Familial transmission rather than defective innate immunity shapes the distinct intestinal microbiota of TLR-deficient mice. J. Exp. Med. 209, 1445–1456 (2012).
Shanahan, M. T. et al. Mouse Paneth cell antimicrobial function is independent of Nod2. Gut http://dx.doi.org/10.1136/gutjnl-2012-304190 (2013).
Robertson, S. J. et al. Nod1 and Nod2 signaling does not alter the composition of intestinal bacterial communities at homeostasis. Gut Microbes 4, 222–231 (2013).
McCafferty, J. et al. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. http://dx.doi.org/10.1038/ismej.2013.106 (2013).
Khazaie, K. et al. Abating colon cancer polyposis by Lactobacillus acidophilus deficient in lipoteichoic acid. Proc. Natl Acad. Sci. USA 109, 10462–10467 (2012).
Acknowledgements
R.F.S. was supported by grants from the US National Institutes of Health (NIH) U54CA163111, R01DK076920 and R01AA020211. C.J. acknowledges support from the NIH (RO1DK047700 and RO1DK073338). The authors thank D. Dapito for critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Adaptive immune responses
-
As opposed to innate immunity, adaptive immune responses are specific to the type of pathogen that is encountered, thereby providing a tailored (albeit slower) immune response. This acquired response is typically mediated by B and T cells with the subsequent generation of memory cells.
- Bacteriocins
-
Antimicrobial peptides released by bacteria to inhibit growth of similar or closely related microorganisms.
- Commensalism
-
A relationship between two organisms in which one organism benefits, whereas the other does not.
- Dysbiosis
-
A state of microbial composition that is characterized by an unbalanced proportion of bacteria compared with the proportion in a healthy state.
- Eubiosis
-
A state of microbial composition in which population abundances are found in normal proportions and typically associated with healthy individuals.
- Facultative anaerobic bacteria
-
Bacteria that are able to generate energy (ATP) through aerobic respiration (electron transport chain) or through fermentation, depending on the amount of oxygen or fermentable products available.
- Germ-free animals
-
Animals born and raised in a sterile environment; they lack any microorganisms (except endogenous viruses).
- Gnotobiotic
-
Describes an animal with a defined microbial population. These animals are born germ-free and then known microorganisms are introduced; this requires that the animals are housed in isolation, to maintain their defined microbial status.
- Horizontal gene transfer
-
The movement of genetic material from one organism to another, without the need for cell division.
- Innate immunity
-
An immune response that recognizes conserved microbial structures, typically through the action of pattern recognition receptors expressed on host cells.
- Metagenome
-
The collection of genomes from members of a specific microbiota.
- Microorganism-associated molecular patterns
-
(MAMPs). Conserved structural components such as lipopolysaccharide, flagellin and nucleic acids derived from microorganisms that are detected by the host innate immune system.
- Muramyl dipeptide
-
A peptidoglycan derivative that is common to both Gram-positive and Gram-negative bacterial cell walls and that triggers an innate immune response.
- Mutualism
-
A relationship between two organisms, in which both organisms benefit.
- Obligate anaerobic bacteria
-
Bacteria that grow without the need for oxygen.
- Parasitism
-
A relationship in which one organism (pathogen) benefits at the expense of another organism.
- Pathobionts
-
Normally innocuous microorganisms that can behave like pathogens if their abundance increases and/or their environmental conditions change.
- Stratum corneum
-
The outermost layer of the epidermis that forms the protective layer of the skin.
- Toll-like receptor
-
(TLR). A family of evolutionarily conserved receptors that recognize microorganism-associated molecular patterns such as flagellin, lipopolysaccharide or nucleic acids. These receptors have an essential role in innate immune responses.
- Tumour tolerance
-
A state of immune hyporesponsiveness, in which tumour antigens induce T cell tolerance (a process that allows tumour immune evasion).
- Virulence factors
-
Molecules expressed by pathogenic microorganisms that help them to gain a growth advantage in a specific ecosystem. These molecules are often responsible for disease manifestation in the host.
Rights and permissions
About this article
Cite this article
Schwabe, R., Jobin, C. The microbiome and cancer. Nat Rev Cancer 13, 800–812 (2013). https://doi.org/10.1038/nrc3610
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3610
This article is cited by
-
Dental health and lung cancer risk in the Golestan Cohort Study
BMC Cancer (2024)
-
NMGMDA: a computational model for predicting potential microbe–drug associations based on minimize matrix nuclear norm and graph attention network
Scientific Reports (2024)
-
Camouflaging attenuated Salmonella by cryo-shocked macrophages for tumor-targeted therapy
Signal Transduction and Targeted Therapy (2024)
-
Composition of the colon microbiota in the individuals with inflammatory bowel disease and colon cancer
Folia Microbiologica (2024)
-
Compositional and functional changes in the salivary microbiota related to oral leukoplakia and oral squamous cell carcinoma: a case control study
BMC Oral Health (2023)