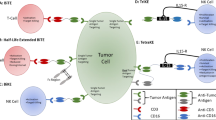
Abstract
Although cancer cells can be immunogenic, tumour progression is associated with the evasion of immunosurveillance, the promotion of tumour tolerance and even the production of pro-tumorigenic factors by immune cells. Cytotoxic T lymphocyte-associated antigen 4 (CTLA4) represents a crucial immune checkpoint, the blockade of which can potentiate anti-tumour immunity. CTLA4-blocking antibodies are now an established therapeutic approach for malignant melanoma, and clinical trials with CTLA4-specific antibodies in prostate cancer have also shown clinical activity. This treatment may provide insights into the targets that the immune system recognizes to drive tumour regression, and could potentially improve both outcome and toxicity for patients with prostate cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
American Cancer Society. Cancer Facts & Figures. American Cancer Society [online], http://www.cancer.org/Research/CancerFactsFigures/index (2011).
Howlader, N. et al. SEER Cancer Statistics Review 1975–2008 National Cancer Institute [online], http://seer.cancer.gov/csr/1975_2008/ (2011).
Knudsen, K. E. & Scher, H. I. Starving the addiction: new opportunities for durable suppression of AR signaling in prostate cancer. Clin. Cancer Res. 15, 4792–4798 (2009).
Laufer, M., Denmeade, S. R., Sinibaldi, V. J., Carducci, M. A. & Eisenberger, M. A. Complete androgen blockade for prostate cancer: what went wrong? J. Urol. 164, 3–9 (2000).
Cha, E. & Fong, L. Immunotherapy for prostate cancer: biology and therapeutic approaches. J. Clin. Oncol. 29, 3677–3685 (2011).
Madan, R. A., Arlen, P. M., Mohebtash, M., Hodge, J. W. & Gulley, J. L. Prostvac-VF: a vector-based vaccine targeting PSA in prostate cancer. Expert Opin. Investig Drugs 18, 1001–1011 (2009).
Gupta, S., Carballido, E. & Fishman, M. Sipuleucel-T for therapy of asymptomatic or minimally symptomatic, castrate-refractory prostate cancer: an update and perspective among other treatments. Onco. Targets Ther. 4, 79–96 (2011).
Akhtar, N. H., Pail, O., Saran, A., Tyrell, L. & Tagawa, S. T. Prostate-specific membrane antigen-based therapeutics. Adv. Urol. 2012, 973820 (2012).
Antonarakis, E. S. & Drake, C. G. Current status of immunological therapies for prostate cancer. Curr. Opin. Urol. 20, 241–246 (2010).
Clive, K. S. et al. Use of GM-CSF as an adjuvant with cancer vaccines: beneficial or detrimental? Expert Rev. Vaccines 9, 519–525 (2010).
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Schaer, D. A., Cohen, A. D. & Wolchok, J. D. Anti-GITR antibodies--potential clinical applications for tumor immunotherapy. Curr. Opin. Investig Drugs 11, 1378–1386 (2010).
Gerritsen, W. et al. A dose-escalation trial of GM-CSF-gene transduced allogeneic prostate cancer cellular immunotherapy in combination with a fully human anti-CTLA antibody (MDX-010, ipilimumab) in patients with metastatic hormone-refractory prostate cancer (mHRPC). J. Clin. Oncol. Abstr. 24 2500 (2006).
Ledford, H. Melanoma drug wins US approval. Nature 471, 561 (2011).
Brunet, J. F. et al. A new member of the immunoglobulin superfamily — CTLA-4. Nature 328, 267–270 (1987).
Peach, R. J. et al. Complementarity determining region 1 (CDR1)- and CDR3-analogous regions in CTLA-4 and CD28 determine the binding to B7–1 J. Exp. Med. 180, 2049–2058 (1994).
Stein, P. H., Fraser, J. D. & Weiss, A. The cytoplasmic domain of CD28 is both necessary and sufficient for costimulation of interleukin-2 secretion and association with phosphatidylinositol 3'-kinase. Mol. Cell Biol. 14, 3392–3402 (1994).
Chuang, E. et al. The CD28 and CTLA-4 receptors associate with the serine/threonine phosphatase PP2A. Immunity 13, 313–322 (2000).
Dariavach, P., Mattei, M. G., Golstein, P. & Lefranc, M. P. Human Ig superfamily CTLA-4 gene: chromosomal localization and identity of protein sequence between murine and human CTLA-4 cytoplasmic domains. Eur. J. Immunol. 18, 1901–1905 (1988).
Schwartz, R. H. Costimulation of T lymphocytes: the role of CD28, CTLA-4, and B7/BB1 in interleukin-2 production and immunotherapy. Cell 71, 1065–1068 (1992).
Linsley, P. S. et al. Binding of the B cell activation antigen B7 to CD28 costimulates T cell proliferation and interleukin 2 mRNA accumulation. J. Exp. Med. 173, 721–730 (1991).
Linsley, P. S. et al. Coexpression and functional cooperation of CTLA-4 and CD28 on activated T lymphocytes. J. Exp. Med. 176, 1595–1604 (1992).
Linsley, P. S. et al. Intracellular trafficking of CTLA-4 and focal localization towards sites of TCR engagement. Immunity 4, 535–543 (1996).
Chuang, E. et al. Interaction of CTLA-4 with the clathrin-associated protein AP50 results in ligand-independent endocytosis that limits cell surface expression. J. Immunol. 159, 144–151 (1997).
van der Merwe, P. A., Bodian, D. L., Daenke, S., Linsley, P. & Davis, S. J. CD80 (B7–1) binds both CD28 and CTLA-4 with a low affinity and very fast kinetics. J. Exp. Med. 185, 393–403 (1997).
Waterhouse, P. et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science 270, 985–988 (1995).
Krummel, M. F. & Allison, J. P. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J. Exp. Med. 183, 2533–2540 (1996).
Masteller, E. L., Chuang, E., Mullen, A. C., Reiner, S. L. & Thompson, C. B. Structural analysis of CTLA-4 function in vivo. J. Immunol. 164, 5319–5327 (2000).
Martin, M., Schneider, H., Azouz, A. & Rudd, C. E. Cytotoxic T lymphocyte antigen 4 and CD28 modulate cell surface raft expression in their regulation of T cell function. J. Exp. Med. 194, 1675–1681 (2001).
Schneider, H. et al. Reversal of the TCR stop signal by CTLA-4. Science 313, 1972–1975 (2006).
Wing, K., Yamaguchi, T. & Sakaguchi, S. Cell-autonomous and -non-autonomous roles of CTLA-4 in immune regulation. Trends Immunol. 32, 428–433 (2011).
Wing, K. & Sakaguchi, S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature Immunol. 11, 7–13 (2010).
Takahashi, T. et al. Immunologic self-tolerance maintained by CD25+CD4+ regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J. Exp. Med. 192, 303–310 (2000).
Read, S. et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J. Immunol. 177, 4376–4383 (2006).
Jain, N., Nguyen, H., Chambers, C. & Kang, J. Dual function of CTLA-4 in regulatory T cells and conventional T cells to prevent multiorgan autoimmunity. Proc. Natl Acad. Sci. USA 107, 1524–1528 (2010).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Kearney, E. R. et al. Antigen-dependent clonal expansion of a trace population of antigen-specific CD4+ T cells in vivo is dependent on CD28 costimulation and inhibited by CTLA-4. J. Immunol. 155, 1032–1036 (1995).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Foster, B. A., Gingrich, J. R., Kwon, E. D., Madias, C. & Greenberg, N. M. Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res. 57, 3325–3330 (1997).
Kwon, E. D. et al. Manipulation of T cell costimulatory and inhibitory signals for immunotherapy of prostate cancer. Proc. Natl Acad. Sci. USA 94, 8099–8103 (1997).
Kwon, E. D. et al. Elimination of residual metastatic prostate cancer after surgery and adjunctive cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) blockade immunotherapy. Proc. Natl Acad. Sci. USA 96, 15074–15079 (1999).
Hurwitz, A. A. et al. Combination immunotherapy of primary prostate cancer in a transgenic mouse model using CTLA-4 blockade. Cancer Res. 60, 2444–2448 (2000).
Hurwitz, A. A., Yu, T. F., Leach, D. R. & Allison, J. P. CTLA-4 blockade synergizes with tumor-derived granulocyte-macrophage colony-stimulating factor for treatment of an experimental mammary carcinoma. Proc. Natl Acad. Sci. USA 95, 10067–10071 (1998).
Mokyr, M. B., Kalinichenko, T., Gorelik, L. & Bluestone, J. A. Realization of the therapeutic potential of CTLA-4 blockade in low-dose chemotherapy-treated tumor-bearing mice. Cancer Res. 58, 5301–5304 (1998).
Demaria, S. et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin. Cancer Res. 11, 728–734 (2005).
Matsumura, S. et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J. Immunol. 181, 3099–3107 (2008).
Ma, Y. et al. Chemotherapy and radiotherapy: cryptic anticancer vaccines. Semin. Immunol. 22, 113–124 (2010).
Drake, C. G. et al. Androgen ablation mitigates tolerance to a prostate/prostate cancer-restricted antigen. Cancer Cell 7, 239–249 (2005).
Sutherland, J. S. et al. Activation of thymic regeneration in mice and humans following androgen blockade. J. Immunol. 175, 2741–2753 (2005).
Perrin, P. J., Maldonado, J. H., Davis, T. A., June, C. H. & Racke, M. K. CTLA-4 blockade enhances clinical disease and cytokine production during experimental allergic encephalomyelitis. J. Immunol. 157, 1333–1336 (1996).
Luhder, F., Hoglund, P., Allison, J. P., Benoist, C. & Mathis, D. Cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) regulates the unfolding of autoimmune diabetes. J. Exp. Med. 187, 427–432 (1998).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Keler, T. et al. Activity and safety of CTLA-4 blockade combined with vaccines in cynomolgus macaques. J. Immunol. 171, 6251–6259 (2003).
Small, E. J. et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin. Cancer Res. 13, 1810–1815 (2007).
Fong, L. et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 69, 609–615 (2009).
Harzstark, A. L. et al. Final results of a phase I study of CTLA-4 blockade in combination with GM-CSF for metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. Abstr. 28, 4689 (2010).
Gerritsen, W. et al. Expanded phase I combination trial of GVAX immunotherapy for prostate cancer and ipilimumab in patients with metastatic hormone-refractory prostate cancer (mHPRC). J. Clin. Oncol. Abstr. 26, 5146 (2008).
Santegoets, S. et al. Lymphoid and myeloid biomarkers for clinical outcome of ipilimumab and prostate GVAX treatment: tumor-related CTLA-4 expression by CD4+ T cells as a dominant predictor of survival. J. Immunother. Abstr. 34, 9 (2011).
Kantoff, P. W. et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Mohebtash, M. et al. Phase I trial of targeted therapy with PSA-TRICOM vaccine (V) and ipilimumab (ipi) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. Abstr. 27, 5144 (2009).
Beer, T. M. et al. Phase I trial of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration resistant prostate cancer (mCRPC). J. Clin. Oncol. Abstr. 26, 5004 (2008).
Slovin, S. F. et al. Initial phase II experience of ipilimumab (IPI) alone and in combination with radiotherapy (XRT) in patients with metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. Abstr. 27, 5138 (2009).
Small, E. J. et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer. J. Clin. Oncol. Abstr. 24, 4609 (2006).
Tollefson, M. K. et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer. 2010 Genitourinary Cancers Symp. Abstr. 168 (2010).
Lang, J. M., Staab, M. J., Liu, G., Wilding, G. & McNeel, D. G. Phase I dose-escalation trial of tremelimumab in combination with bicalutamide in patients with recurrent prostate cancer. J. Clin. Oncol. Abstr. 29, 174 (2011).
Robert, C. et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N. Engl. J. Med. 364, 2517–2526 (2011).
Wolchok, J. D. et al. Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol. 11, 155–164 (2010).
Madan, R. A. et al. Overall survival (OS) analysis of a phase I trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab (Ipi) in the treatment of metastatic castration-resistant prostate cancer (mCRPC). J. Clin. Oncol. Abstr. 28, 2550 (2010).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Comin-Anduix, B. et al. Detailed analysis of immunologic effects of the cytotoxic T lymphocyte-associated antigen 4-blocking monoclonal antibody tremelimumab in peripheral blood of patients with melanoma. J. Transl. Med. 6, 22 (2008).
Liakou, C. I. et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc. Natl Acad. Sci. USA 105, 14987–14992 (2008).
Phan, G. Q. et al. Cancer regression and autoimmunity induced by cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma. Proc. Natl Acad. Sci. USA 100, 8372–8377 (2003).
Kavanagh, B. et al. CTLA4 blockade expands FoxP3+ regulatory and activated effector CD4+ T cells in a dose-dependent fashion. Blood 112, 1175–1183 (2008).
Quezada, S. A., Peggs, K. S., Curran, M. A. & Allison, J. P. CTLA4 blockade and GM-CSF combination immunotherapy alters the intratumor balance of effector and regulatory T cells. J. Clin. Invest. 116, 1935–1945 (2006).
Mitsui, J. et al. Two distinct mechanisms of augmented antitumor activity by modulation of immunostimulatory/inhibitory signals. Clin. Cancer Res. 16, 2781–2791 (2010).
Fasso, M. et al. SPAS-1 (stimulator of prostatic adenocarcinoma-specific T cells)/SH3GLB2: A prostate tumor antigen identified by CTLA-4 blockade. Proc. Natl Acad. Sci. USA 105, 3509–3514 (2008).
Lapointe, J. et al. Gene expression profiling identifies clinically relevant subtypes of prostate cancer. Proc. Natl Acad. Sci. USA 101, 811–816 (2004).
Yuan, J. et al. CTLA-4 blockade enhances polyfunctional NY-ESO-1 specific T cell responses in metastatic melanoma patients with clinical benefit. Proc. Natl Acad. Sci. USA 105, 20410–20415 (2008).
Dubovsky, J. A., Albertini, M. R. & McNeel, D. G. MAD-CT-2 identified as a novel melanoma cancer-testis antigen using phage immunoblot analysis. J. Immunother. 30, 675–683 (2007).
Goff, S. L., Robbins, P. F., El-Gamil, M. & Rosenberg, S. A. No correlation between clinical response to CTLA-4 blockade and presence of NY-ESO-1 antibody in patients with metastatic melanoma. J. Immunother. 32, 884–885 (2009).
Yuan, J. et al. Integrated NY-ESO-1 antibody and CD8+ T-cell responses correlate with clinical benefit in advanced melanoma patients treated with ipilimumab. Proc. Natl Acad. Sci. USA 108, 16723–16728 (2011).
Jinushi, M., Hodi, F. S. & Dranoff, G. Therapy-induced antibodies to MHC class I chain-related protein A antagonize immune suppression and stimulate antitumor cytotoxicity. Proc. Natl Acad. Sci. USA 103, 9190–9195 (2006).
Schoenfeld, J. et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 70, 10150–10160 (2010).
Chesney, J. et al. An essential role for macrophage migration inhibitory factor (MIF) in angiogenesis and the growth of a murine lymphoma. Mol. Med. 5, 181–191 (1999).
Fong, L. et al. Identification of novel prostate cancer-associated antigens through antibody profiling of prostate cancer patients treated with CTLA-4 blockade. J. Clin. Oncol. Abstr. 28, 2578 (2010).
Nesslinger, N. J. et al. Standard treatments induce antigen-specific immune responses in prostate cancer. Clin. Cancer Res. 13, 1493–1502 (2007).
Morse, M. D. & McNeel, D. G. Prostate cancer patients on androgen deprivation therapy develop persistent changes in adaptive immune responses. Hum. Immunol. 71, 496–504 (2010).
Acknowledgements
S.S.K. is supported by a Peter Michael Foundation Pelican Fellowship. E.C. is supported by an ASCO Young Investivator Award. L.F. is supported by NIH R01 CA136753 and the Prostate Cancer Foundation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
DATABASES
FURTHER INFORMATION
Rights and permissions
About this article
Cite this article
Kwek, S., Cha, E. & Fong, L. Unmasking the immune recognition of prostate cancer with CTLA4 blockade. Nat Rev Cancer 12, 289–297 (2012). https://doi.org/10.1038/nrc3223
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3223
This article is cited by
-
Telomerase-based GX301 cancer vaccine in patients with metastatic castration-resistant prostate cancer: a randomized phase II trial
Cancer Immunology, Immunotherapy (2021)
-
Potentiating prostate cancer immunotherapy with oncolytic viruses
Nature Reviews Urology (2018)
-
Radiotherapy and the abscopal effect: insight from the past, present, and future
Journal of Radiation Oncology (2015)
-
Synthetic immunity to break down the bottleneck of cancer immunotherapy
Science Bulletin (2015)
-
Dysfunction of PSA-specific CD8+ T cells in prostate cancer patients correlates with CD38 and Tim-3 expression
Cancer Immunology, Immunotherapy (2015)