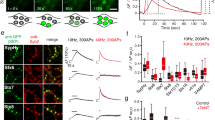
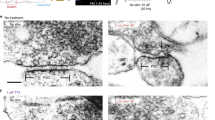
Abstract
Fast activity-driven turnover of neurotransmitter-filled vesicles at presynaptic terminals is a crucial step in information transfer in the CNS. Characterization of the relationship between the nanoscale organization of synaptic vesicles and their functional properties during transmission is currently of interest. Here we outline a procedure for ultrastructural investigation of functional vesicles in synapses from native mammalian brain tissue. FM dye is injected into the target region of a brain slice and upstream axons are electrically activated to stimulate vesicle turnover and dye uptake. In the presence of diaminobenzidine (DAB), photoactivation of dye-filled vesicles yields an osmiophilic precipitate that is visible in electron micrographs. When combined with serial-section electron microscopy, fundamental ultrastructure-function relationships of presynaptic terminals in native circuits are revealed. We outline the utility of this protocol for the 3D reconstruction of a recycling vesicle pool in CA3–CA1 synapses from an acute hippocampal slice and for the characterization of its anatomically defined docked pool. This protocol requires 6–7 d.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Sudhof, T.C. The synaptic vesicle cycle. Annu. Rev. Neurosci. 27, 509–547 (2004).
Alabi, A.A. et al. Synaptic vesicle pools and dynamics. Cold Spring Harb. Perspect. Biol. 4, a013680 (2012).
Rizzoli, S.O. et al. Synaptic vesicle pools. Nat. Rev. Neurosci. 6, 57–69 (2005).
Rosenmund, C. et al. Definition of the readily releasable pool of vesicles at hippocampal synapses. Neuron 16, 1197–1207 (1996).
Fredj, N.B. et al. A resting pool of vesicles is responsible for spontaneous vesicle fusion at the synapse. Nat. Neurosci. 12, 751–758 (2009).
Sara, Y. et al. An isolated pool of vesicles recycles at rest and drives spontaneous neurotransmission. Neuron 45, 563–573 (2005).
Staras, K. et al. A vesicle superpool spans multiple presynaptic terminals in hippocampal neurons. Neuron 66, 37–44 (2010).
Westphal, V. et al. Video-rate far-field optical nanoscopy dissects synaptic vesicle movement. Science 320, 246–249 (2008).
Kim, S.H. et al. CDK5 serves as a major control point in neurotransmitter release. Neuron 67, 797–809 (2010).
Ratnayaka, A. et al. Recruitment of resting vesicles into recycling pools supports NMDA-receptor dependent synaptic potentiation in cultured hippocampal neurons. J. Physiol. 590, 1585–1597 (2012).
Murthy, V.N. et al. Inactivity produces increases in neurotransmitter release and synapse size. Neuron 32, 673–682 (2001).
Thiagarajan, T.C. et al. Adaptation to synaptic inactivity in hippocampal neurons. Neuron 47, 725–737 (2005).
Tyler, W.J. et al. BDNF increases release probability and the size of a rapidly recycling vesicle pool within rat hippocampal excitatory synapses. J. Physiol. 574, 787–803 (2006).
Staras, K. Share and share alike: trading of presynaptic elements between central synapses. Trends Neurosci. 30, 292–298 (2007).
Staras, K. et al. Sharing vesicles between central presynaptic terminals: implications for synaptic function. Front Synaptic Neurosci. 2, 20 (2010).
Murphy, D.D. et al. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J. Neurosci. 20, 3214–3220 (2000).
Scott, D. et al. α-Synuclein inhibits intersynaptic vesicle mobility and maintains recycling-pool homeostasis. J. Neurosci. 32, 10129–10135 (2012).
Vos, M. et al. Synaptic mitochondria in synaptic transmission and organization of vesicle pools in health and disease. Front Synaptic Neurosci. 2, 139 (2010).
Schweizer, F.E. et al. The synaptic vesicle: cycle of exocytosis and endocytosis. Curr. Opin. Neurobiol. 16, 298–304 (2006).
Zhang, Q. et al. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science 323, 1448–1453 (2009).
Park, H. et al. Influence of synaptic vesicle position on release probability and exocytotic fusion mode. Science 335, 1362–1366 (2012).
Betz, W.J. et al. Optical analysis of synaptic vesicle recycling at the frog neuromuscular junction. Science 255, 200–203 (1992).
Ryan, T.A. et al. The kinetics of synaptic vesicle recycling measured at single presynaptic boutons. Neuron 11, 713–724 (1993).
Gaffield, M.A. et al. Imaging synaptic vesicle exocytosis and endocytosis with FM dyes. Nat. Protoc. 1, 2916–2921 (2006).
Darcy, K.J. et al. Constitutive sharing of recycling synaptic vesicles between presynaptic boutons. Nat. Neurosci. 9, 315–321 (2006).
de Lange, R.P. et al. Two modes of vesicle recycling in the rat calyx of Held. J. Neurosci. 23, 10164–10173 (2003).
Denker, A. et al. A small pool of vesicles maintains synaptic activity in vivo. Proc. Natl. Acad. Sci. USA 108, 17177–17182 (2011).
Denker, A. et al. Revisiting synaptic vesicle pool localization in the Drosophila neuromuscular junction. J. Physiol. 587, 2919–2926 (2009).
Henkel, A.W. et al. FM1-43 dye ultrastructural localization in and release from frog motor nerve terminals. Proc. Natl. Acad. Sci. USA 93, 1918–1923 (1996).
Rizzoli, S.O. et al. The structural organization of the readily releasable pool of synaptic vesicles. Science 303, 2037–2039 (2004).
Schikorski, T. et al. Morphological correlates of functionally defined synaptic vesicle populations. Nat. Neurosci. 4, 391–395 (2001).
Teng, H. et al. Clathrin-mediated endocytosis near active zones in snake motor boutons. J. Neurosci. 20, 7986–7993 (2000).
Harata, N. et al. Visualizing recycling synaptic vesicles in hippocampal neurons by FM 1-43 photoconversion. Proc. Natl. Acad. Sci. USA 98, 12748–12753 (2001).
Branco, T. et al. Examining size-strength relationships at hippocampal synapses using an ultrastructural measurement of synaptic release probability. J. Struct. Biol. 172, 203–210 (2010).
Ratnayaka, A. et al. Extrasynaptic vesicle recycling in mature hippocampal neurons. Nat. Commun. 2, 531 (2011).
Welzel, O. et al. Systematic heterogeneity of fractional vesicle pool sizes and release rates of hippocampal synapses. Biophys. J. 100, 593–601 (2011).
Richards, D.A. et al. Two endocytic recycling routes selectively fill two vesicle pools in frog motor nerve terminals. Neuron 27, 551–559 (2000).
Richards, D.A. et al. Synaptic vesicle pools at the frog neuromuscular junction. Neuron 39, 529–541 (2003).
Paillart, C. et al. Endocytosis and vesicle recycling at a ribbon synapse. J. Neurosci. 23, 4092–4099 (2003).
Marra, V. et al. A preferentially segregated recycling vesicle pool of limited size supports neurotransmission in native central synapses. Neuron 76, 579–589 (2012).
Pyle, J.L. et al. Visualization of synaptic activity in hippocampal slices with FM1-43 enabled by fluorescence quenching. Neuron 24, 803–808 (1999).
Zakharenko, S.S. et al. Visualization of changes in presynaptic function during long-term synaptic plasticity. Nat. Neurosci. 4, 711–717 (2001).
Zakharenko, S.S. et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron 39, 975–990 (2003).
Zakharenko, S.S. et al. Altered presynaptic vesicle release and cycling during mGluR-dependent LTD. Neuron 35, 1099–1110 (2002).
Jensen, F.E. et al. Preservation of neuronal ultrastructure in hippocampal slices using rapid microwave-enhanced fixation. J. Neurosci. Methods 29, 217–230 (1989).
Bischofberger, J. et al. Patch-clamp recording from mossy fiber terminals in hippocampal slices. Nat. Protoc. 1, 2075–2081 (2006).
Debanne, D. et al. Paired-recordings from synaptically coupled cortical and hippocampal neurons in acute and cultured brain slices. Nat. Protoc. 3, 1559–1568 (2008).
Acknowledgements
This work was supported by Wellcome Trust (WT084357MF), Biotechnology and Biological Sciences Research Council (BBSRC) (BB/K019015/1), Medical Research Council (MRC) (MR/K004999/1) and European Union (EU) (FP7-308943) grants to K.S.
Author information
Authors and Affiliations
Contributions
K.S. and V.M. conceived the method and wrote the paper. F.C. validated the key steps and provided some of the figures. J.J.B. helped develop sample processing methods and carried out serial sectioning.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Marra, V., Burden, J., Crawford, F. et al. Ultrastructural readout of functional synaptic vesicle pools in hippocampal slices based on FM dye labeling and photoconversion. Nat Protoc 9, 1337–1347 (2014). https://doi.org/10.1038/nprot.2014.088
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.088
This article is cited by
-
CLARITY-compatible lipophilic dyes for electrode marking and neuronal tracing
Scientific Reports (2016)
-
Ultrastructural and functional fate of recycled vesicles in hippocampal synapses
Nature Communications (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.