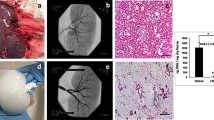
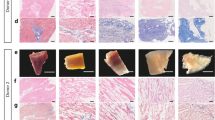
Abstract
The native extracellular matrix (ECM) outlines the architecture of organs and tissues. It provides a unique niche of composition and form, which serves as a foundational scaffold that supports organ-specific cell types and enables normal organ function. Here we describe a standard process for pressure-controlled perfusion decellularization of whole organs for generating acellular 3D scaffolds with preserved ECM protein content, architecture and perfusable vascular conduits. By applying antegrade perfusion of detergents and subsequent washes to arterial vasculature at low physiological pressures, successful decellularization of complex organs (i.e., hearts, lungs and kidneys) can be performed. By using appropriate modifications, pressure-controlled perfusion decellularization can be achieved in small-animal experimental models (rat organs, 4–5 d) and scaled to clinically relevant models (porcine and human organs, 12–14 d). Combining the unique structural and biochemical properties of native acellular scaffolds with subsequent recellularization techniques offers a novel platform for organ engineering and regeneration, for experimentation ex vivo and potential clinical application in vivo.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Hoshiba, T. et al. Decellularized matrices for tissue engineering. Exp. Opin. Biol. Ther. 10, 1717–1728 (2010).
Badylak, S.F., Freytes, D.O. & Gilbert, T.W. Extracellular matrix as a biological scaffold material: structure and function. Acta. Biomater. 5, 1–13 (2009).
Mostow, E.N. et al. Effectiveness of an extracellular matrix graft (OASIS wound matrix) in the treatment of chronic leg ulcers: a randomized clinical trial. J. Vasc. Surg. 41, 837–843 (2005).
Cornwell, K.G., Landsman, A. & James, K.S. Extracellular matrix biomaterials for soft tissue repair. Clin. Podiatr. Med. Surg. 26, 507–523 (2009).
Boyd, W.D. et al. Pericardial reconstruction using an extracellular matrix implant correlates with reduced risk of postoperative atrial fibrillation in coronary artery bypass surgery patients. Heart Surg. Forum 13, E311–E316 (2010).
Dasi, L.P. et al. Fluid mechanics of artificial heart valves. Clin. Exp. Pharmacol. Physiol. 36, 225–237 (2009).
Crapo, P.M., Gilbert, T.W. & Badylak, S.F. An overview of tissue and whole organ decellularization processes. Biomaterials 32, 3233–3243 (2011).
Gilbert, T.W., Sellaro, T.L. & Badylak, S.F. Decellularization of tissues and organs. Biomaterials 27, 3675–3683 (2006).
Burk, J. et al. Freeze-thaw cycles enhance decellularization of large tendons. Tissue Eng. Part C Methods 20, 276–284 (2013).
Nonaka, P.N. et al. Effects of freezing/thawing on the mechanical properties of decellularized lungs. J. Biomed. Mater. Res. A 102, 413–419 (2014).
Wainwright, J.M. et al. Preparation of cardiac extracellular matrix from an intact porcine heart. Tissue Eng. Part C Methods 16, 525–532 (2010).
Yang, M. et al. Favorable effects of the detergent and enzyme extraction method for preparing decellularized bovine pericardium scaffold for tissue engineered heart valves. J. Biomed. Mater. Res. B Appl. Biomater. 91, 354–361 (2009).
Rieder, E. et al. Decellularization protocols of porcine heart valves differ importantly in efficiency of cell removal and susceptibility of the matrix to recellularization with human vascular cells. J. Thorac. Cardiovasc. Surg. 127, 399–405 (2004).
Sullivan, D.C. et al. Decellularization methods of porcine kidneys for whole organ engineering using a high-throughput system. Biomaterials 33, 7756–7764 (2012).
Uygun, B.E. et al. Organ reengineering through development of a transplantable recellularized liver graft using decellularized liver matrix. Nat. Med. 16, 814–820 (2010).
Petersen, T.H. et al. Tissue-engineered lungs for in vivo implantation. Science 329, 538–541 (2010).
Quint, C. et al. Decellularized tissue-engineered blood vessel as an arterial conduit. Proc. Natl. Acad. Sci. USA 108, 9214–9219 (2011).
Price, A.P. et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng. Part A 16, 2581–2591 (2010).
Cebotari, S. et al. Detergent decellularization of heart valves for tissue engineering: toxicological effects of residual detergents on human endothelial cells. Artif. Organs 34, 206–210 (2010).
Mishra, D.K. et al. Human lung cancer cells grown on acellular rat lung matrix create perfusable tumor nodules. Ann. Thorac. Surg. 93, 1075–1081 (2012).
Sokocevic, D. et al. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials 34, 3256–3269 (2013).
Nelson, C.M. & Bissell, M.J. Of extracellular matrix, scaffolds, and signaling: tissue architecture regulates development, homeostasis, and cancer. Annu. Rev. Cell Dev. Biol. 22, 287–309 (2006).
Bornstein, P. & Sage, E.H. Matricellular proteins: extracellular modulators of cell function. Curr. Opin. Cell Biol. 14, 608–616 (2002).
Ofenbauer, A. et al. Dewaxed ECM: A simple method for analyzing cell behaviour on decellularized extracellular matrices. J. Tissue Eng. Regen. Med. 10.1002/term.1658 (2012).
Soto-Gutierrez, A. et al. Perspectives on whole-organ assembly: moving toward transplantation on demand. J. Clin. Invest. 122, 3817–3823 (2012).
Ott, H.C. et al. Regeneration and orthotopic transplantation of a bioartificial lung. Nat. Med. 16, 927–933 (2010).
Song, J.J. et al. Enhanced in vivo function of bioartificial lungs in rats. Ann. Thorac. Surg. 92, 998–1005 (2011).
Song, J.J. et al. Regeneration and experimental orthotopic transplantation of a bioengineered kidney. Nat. Med. 19, 646–651 (2013).
Badylak, S.F., Taylor, D. & Uygun, K. Whole-organ tissue engineering: decellularization and recellularization of three-dimensional matrix scaffolds. Annu. Rev. Biomed. Eng. 13, 27–53 (2011).
Orlando, G. et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann. Surg. 256, 363–370 (2012).
Ott, H.C. et al. Perfusion-decellularized matrix: using nature's platform to engineer a bioartificial heart. Nat. Med. 14, 213–221 (2008).
Lee, L.T., Deas, J.E. & Howe, C. Removal of unbound sodium dodecyl sulfate (SDS) from proteins in solution by electrophoresis through Triton X-100–agarose. J. Immunol. Methods 19, 69–75 (1978).
Goh, S.K. et al. Perfusion-decellularized pancreas as a natural 3D scaffold for pancreatic tissue and whole organ engineering. Biomaterials 34, 6760–6772 (2013).
He, M. & Callanan, A. Comparison of methods for whole-organ decellularization in tissue engineering of bioartificial organs. Tissue Eng. Part B Rev. 19, 194–208 (2013).
Jensen, T. et al. A rapid lung de-cellularization protocol supports embryonic stem cell differentiation in vitro and following implantation. Tissue Eng. Part C Methods 18, 632–646 (2012).
Nichols, J.E. et al. Production and assessment of decellularized pig and human lung scaffolds. Tissue Eng. Part A 19, 2045–2062 (2013).
Gilpin, S.E. et al. Perfusion decellularization of human and porcine lungs: Bringing the matrix to clinical scale. J. Heart Lung Transplant. 33, 298–308 (2013).
Akhyari, P. et al. The quest for an optimized protocol for whole-heart decellularization: a comparison of three popular and a novel decellularization technique and their diverse effects on crucial extracellular matrix qualities. Tissue Eng. Part C Methods 17, 915–926 (2011).
Wallis, J.M. et al. Comparative assessment of detergent-based protocols for mouse lung de-cellularization and re-cellularization. Tissue Eng. Part C Methods 18, 420–432 (2012).
Ren, H. et al. Evaluation of two decellularization methods in the development of a whole-organ decellularized rat liver scaffold. Liver Int. 33, 448–458 (2013).
Venkatasubramanian, R.T. et al. Effects of freezing and cryopreservation on the mechanical properties of arteries. Ann. Biomed. Eng. 34, 823–832 (2006).
Venkatasubramanian, R.T. et al. Freeze-thaw induced biomechanical changes in arteries: role of collagen matrix and smooth muscle cells. Ann. Biomed. Eng. 38, 694–706 (2010).
Cortiella, J. et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng. Part A 16, 2565–2580 (2010).
Lu, T.Y. et al. Repopulation of decellularized mouse heart with human induced pluripotent stem cell-derived cardiovascular progenitor cells. Nat. Commun. 4, 2307 (2013).
O'Neill, J.D. et al. Decellularization of human and porcine lung tissues for pulmonary tissue engineering. Ann. Thorac. Surg. 96, 1046–1055 (2013).
Bonvillain, R.W. et al. A nonhuman primate model of lung regeneration: detergent-mediated decellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng. Part A 18, 2437–2452 (2012).
Lahteenmaki, K. et al. Bacterial plasminogen receptors: in vitro evidence for a role in degradation of the mammalian extracellular matrix. Infect Immun. 63, 3659–3664 (1995).
Okamoto, T. et al. Activation of human matrix metalloproteinases by various bacterial proteinases. J. Biol. Chem. 272, 6059–6066 (1997).
Kemp, P.D. Peracetic Acid Sterilization of Collagen or Collagenous Tissue U.P. Office, Organogenesis Inc. (1995).
Rusconi, F. et al. Quantification of sodium dodecyl sulfate in microliter-volume biochemical samples by visible light spectroscopy. Anal. Biochem. 295, 31–37 (2001).
Terada, H. et al. Quantitative determination by derivative spectrophotometry of Triton X-100 in solubilized preparations of membrane proteins. Anal. Biochem. 149, 501–506 (1985).
Yang, B. et al. Development of a porcine bladder acellular matrix with well-preserved extracellular bioactive factors for tissue engineering. Tissue Eng. Part C Methods 16, 1201–1211 (2010).
Jungebluth, P. et al. Structural and morphologic evaluation of a novel detergent-enzymatic tissue-engineered tracheal tubular matrix. J. Thorac. Cardiovasc. Surg. 138, 586–593 (2009).
Conrad, C. et al. Bio-engineered endocrine pancreas based on decellularized pancreatic matrix and mesenchymal stem cell/islet cell coculture. Am. Coll. of Surgeons 211, 62 (2010).
Acknowledgements
The present study was supported by the US National Institutes of Health (NIH) Director's New Innovator Award DP2 OD008749-01, grants R21 HL108663-01 and R01 HL108678, by seed grants by the Department of Surgery, Massachusetts General Hospital and the Harvard Stem Cell Institute, and a research grant by United Therapeutics Inc. We thank D.J. Mathisen and J.P. Vacanti for their mentorship and senior advice on surgical procedures, tissue engineering aspects and for critical review of the manuscript.
Author information
Authors and Affiliations
Contributions
H.C.O. conceived and designed the initial protocol, and oversaw data collection and writing of the manuscript. J.P.G. provided further development of heart and kidney decellularization protocols, oversaw data analysis and figure creation, and contributed to the manuscript and review. S.E.G. provided further development of lung and kidney decellularization protocols, oversaw data analysis and figure creation, and contributed to the manuscript and review. J.M.C. provided biochemical and histological data characterization, oversaw figure creation, and contributed to the manuscript. L.F.T. provided biochemical and histological data characterization, and contributed to manuscript and revisions. X.R. provided characterization of decellularized organ vasculature.
Corresponding author
Ethics declarations
Competing interests
H.C.O. is the founder and stockholder of IVIVA Medical, Inc. This relationship did not affect the content or conclusions contained in this manuscript.
Supplementary information
Supplementary Methods
Immunohistochemistry and biochemical analysis. (PDF 106 kb)
Rights and permissions
About this article
Cite this article
Guyette, J., Gilpin, S., Charest, J. et al. Perfusion decellularization of whole organs. Nat Protoc 9, 1451–1468 (2014). https://doi.org/10.1038/nprot.2014.097
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.097
This article is cited by
-
Capturing effects of blood flow on the transplanted decellularized nephron with intravital microscopy
Scientific Reports (2023)
-
An efficient strategy to recellularization of a rat aorta scaffold: an optimized decellularization, detergent removal, and Apelin-13 immobilization
Biomaterials Research (2022)
-
3D Printing: Advancement in Biogenerative Engineering to Combat Shortage of Organs and Bioapplicable Materials
Regenerative Engineering and Translational Medicine (2022)
-
Perfusion-Based Recellularization of Rat Livers with Islets of Langerhans
Journal of Medical and Biological Engineering (2022)
-
Towards organoid culture without Matrigel
Communications Biology (2021)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.