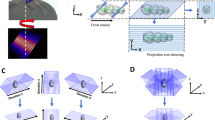
Abstract
We describe the construction and use of a compact dual-view inverted selective plane illumination microscope (diSPIM) for time-lapse volumetric (4D) imaging of living samples at subcellular resolution. Our protocol enables a biologist with some prior microscopy experience to assemble a diSPIM from commercially available parts, to align optics and test system performance, to prepare samples, and to control hardware and data processing with our software. Unlike existing light sheet microscopy protocols, our method does not require the sample to be embedded in agarose; instead, samples are prepared conventionally on glass coverslips. Tissue culture cells and Caenorhabditis elegans embryos are used as examples in this protocol; successful implementation of the protocol results in isotropic resolution and acquisition speeds up to several volumes per s on these samples. Assembling and verifying diSPIM performance takes ∼6 d, sample preparation and data acquisition take up to 5 d and postprocessing takes 3–8 h, depending on the size of the data.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Accession codes
References
Voie, A.H., Burns, D.H. & Spelman, F.A. Orthogonal-plane fluorescence optical sectioning: three-dimensional imaging of macroscopic biological specimens. J. Microsc. 170, 229–236 (1993).
Fuchs, E., Jaffe, J.S., Long, R.A. & Azam, F. Thin laser light sheet microscope for microbial oceanography. Opt. Express 10, 145–154 (2002).
Huisken, J., Swoger, J., Del Bene, F., Wittbrodt, J. & Stelzer, E.H.K. Optical sectioning deep inside live embryos by selective plane illumination microscopy. Science 305, 1007–1009 (2004).
Holekamp, T.F., Turaga, D. & Holy, T.E. Fast three-dimensional fluorescence imaging of activity in neural populations by objective-coupled planar illumination microscopy. Neuron 57, 661–672 (2008).
Wu, Y. et al. Inverted selective plane illumination microscopy (iSPIM) enables coupled cell identity lineaging and neurodevelopmental imaging in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 108, 17708–17713 (2011).
Keller, P.J., Schmidt, A., Wittbrodt, J. & Stelzer, E.H.K. Reconstruction of zebrafish early embryonic development by scanned light sheet microscopy. Science 322, 1065–1069 (2008).
Tomer, R., Khairy, K., Amat, F. & Keller, P.J. Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy. Nat. Methods 9, 755–763 (2012).
Krzic, U., Gunther, S., Saunders, T.E., Streichan, S.J. & Hufnagel, L. Multiview light-sheet microscope for rapid in toto imaging. Nat. Methods 9, 730–733 (2012).
Planchon, T.A. et al. Rapid three-dimensional isotropic imaging of living cells using Bessel beam plane illumination. Nat. Methods 8, 417–423 (2011).
Gao, L. et al. Noninvasive imaging beyond the diffraction limit of 3D dynamics in thickly fluorescent specimens. Cell 151, 1370–1385 (2012).
Wu, Y. et al. Spatially isotropic four-dimensional imaging with dual-view plane illumination microscopy. Nat. Biotechnol. 31, 1032–1038 (2013).
Swoger, J., Verveer, P., Greger, K., Huisken, J. & Stelzer, E.H.K. Multi-view image fusion improves resolution in three-dimensional microscopy. Opt. Express 15, 8029–8042 (2007).
Temerinac-Ott, M. et al. Multiview deblurring for 3D images from light-sheet-based fluorescence microscopy. IEEE Trans Image Process. 21, 1863–1873 (2012).
Pitrone, P.G. et al. OpenSPIM: an open-access light-sheet microscopy platform. Nat. Methods 10, 598–599 (2013).
Gualda, E.J. et al. OpenSpinMicroscopy: an open-source integrated microscopy platform. Nat. Methods 10, 599–600 (2013).
Capoulade, J., Wachsmuth, M., Hufnagel, L. & Knop, M. Quantitative fluorescence imaging of protein diffusion and interaction in living cells. Nat. Biotechnol. 29, 835–839 (2011).
Gao, L., Shao, L., Chen, B.C. & Betzig, E. 3D live fluorescence imaging of cellular dynamics using Bessel beam plane illumination microscopy. Nat. Protoc. 9, 1083–1101 (2014).
Huisken, J. & Stainier, D.Y.R. Even fluorescence excitation by multidirectional selective plane illumination microscopy (mSPIM). Opt. Lett. 32, 2608–2610 (2007).
Silvestri, L., Bria, A., Sacconi, L., Iannello, G. & Pavones, F.S. Confocal light sheet microscopy: micron-scale neuroanatomy of the entire mouse brain. Opt. Express 20, 20582–20598 (2012).
McAuliffe, M.J. et al. Medical image processing, analysis, and visualization in clinical research. Proceedings of the 14th IEEE Symposium on Comput.er-Based Medical Systems (CBMS '01) 381 (2001).
Stiernagle, T. Maintenance of C. elegans. in WormBook (ed. The C. elegans Research Community) 10.1895/wormbook.1.101.1 (11 February 2006).
York, A.G. et al. Instant super-resolution imaging in live cells and embryos via analog image processing. Nat. Methods 10, 1122–1126 (2013).
Murray, J.I . & Bao, Z. in Imaging in Developmental Biology: A Laboratory Manual Vol. 56 (eds. Sharpe, J. & Wong, R.) (Cold Spring Harbor Laboratory, 2010).
Shroff, H. et al. Dual-color superresolution imaging of genetically expressed probes within individual adhesion complexes. Proc. Natl. Acad. Sci. USA 104, 20308–20313 (2007).
Acknowledgements
We thank V. Kopuri and J. Daniels for their help with instrumentation; M. Anthony for the Solidworks drawings; A. Santella for troubleshooting registration issues with the MIPAV plug-in; and B. Mohler, A. York, H. Eden and J.-Bernard Fiche for their critical feedback on the diSPIM system. We also thank the Research Center for Minority Institutions program and the Institute of Neurobiology at the University of Puerto Rico for providing a meeting and brainstorming platform. This work was partially conducted at the Marine Biological Laboratories at Woods Hole, under a Whitman research award (to D.A.C.-R., Z.B. and H.S.). This work was supported by the Intramural Research Programs of the NIH National Institute of Biomedical Imaging and Bioengineering, the Center for Information Technology and by NIH grants U01 HD075602 and OD016474. The NIH does not endorse or recommend any commercial products, processes or services. The views and opinions of authors expressed here do not necessarily state or reflect those of the U.S. Government, and they may not be used for advertising and product endorsement purposes. Links to internet sites are only for the convenience of readers. The NIH is not responsible for the availability or the content of these external sites, nor does NIH endorse, warrant or guarantee the products, services or information described or offered at these internet sites.
Author information
Authors and Affiliations
Contributions
A.K., G.R., Y.W. and H.S. designed and built the optical system; A.K., G.R. and Y.W. designed the control electronics; Y.W., W.G., E.M., A.B. and M.M. designed the registration, fusion and deconvolution of the GPU-based software; A.K., Y.W., G.R., R.C., P.C. and H.S. tested the system; A.K., R.C. and P.C. performed the experiments; Z.B., D.A.C.-R. and H.S. supervised the research; and A.K., G.R., Y.W., R.C., P.C. and H.S. wrote the paper.
Corresponding authors
Ethics declarations
Competing interests
G.R. declares a competing financial interest as an employee of ASI.
Integrated supplementary information
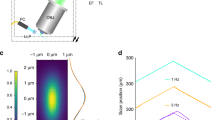
Supplementary Figure 1 Diagnostics for assessing excitation input to diSPIM.
a. After projecting the excitation beam through the scanner onto a far screen or wall (approximately three feet from the output of the scanner, without mounting the tube lens), the beam should fill the image of the 2D MEMS mirror (right). An example of a misaligned beam is shown at left: note that the MEMS mirror is underfilled and the beam spills off the MEMS aperture. b. When checking centration and collimation of the beams through the scanner + tube lens assemblies, we mount them together on the bracket (ASI, Cat. # B1013 & B1034) as shown. c. Attaching a circular piece of white paper (2 inches in diameter) to the output of each tube lens is helpful in assessing beam centration. d. When properly collimated, both beams should maintain their size, and appear the same size at a screen placed far from the tube lens face (here a wall 12 feet from the output of each tube lens was used to assess mutual beam size).
Supplementary Figure 2 Offset and aligned SPIM beams as viewed in the bottom camera.
Top: offset beams from each arm, as viewed through bottom imaging path (with the 10x objective), on a glass coverslip coated with yellow-green fluorescent beads. Red arrows indicate beam waist, blue arrows indicate reflections of each beam from coverslip (laterally displaced from the beam waist). Bottom: beams are aligned after adjusting objective adjuster. Note that beams have not been scanned to create the light sheet. Scalebars: 200 μm.
Supplementary Figure 3 'Pencil' and epi beams as viewed in SPIM camera.
The pencil beam produced by one arm, as viewed in the other arm's SPIM camera. The epi beam is also indicated. Dye has been added to the imaging chamber in order to enable visualization of the excitation. Scalebar: 40 μm.
Supplementary Figure 4 Fine adjustment of light sheets, as viewed in the bottom camera.
a. The left-most sheet is tilted. b. Rotating the scanner associated with this sheet corrects the tilt, but the two sheets are offset vertically with respect to each other. c. Adjusting the 'Y galvo offset' parameter in the diSPIM Control.vi correctly aligns the vertical offset of the two sheets, but they are still offset in the horizontal direction. d. Bringing the diSPIM module down, towards the coverslip, moves the sheets together until they are aligned. The sample is a coverslip coated with yellow-green fluorescent beads. Scalebars: 200 μm.
Supplementary Figure 5 Epifluorescence, SPIM, and overlapped beams as viewed in arm A camera.
Top: Epifluorescence signal detected in arm A, when illuminating with sheet generated by arm A. Middle: Fluorescence detected by arm A, when illuminating with sheet generated by arm B (laser input to arm A is blocked). Bottom: The two sheets are aligned when the epifluorescence and SPIM signals overlap. The sample is a coverslip coated with yellow-green fluorescent beads. Scalebars: 50 μm.
Supplementary Figure 6 Misalignment in diSPIM components diagnosed through arm A camera.
All images show both epifluorescence signal detected in arm A when illuminating with the sheet generated by arm A, and fluorescence signal detected in arm A when illuminating with the sheet generated by arm B. When all system components are aligned, the two fluorescence signals should overlap, resulting in a crisp image of the fluorescent beads (left column). The other panels show various misalignments, causing a blurry fluorescence signal resulting from the sheet generated by arm B (the epifluorescence signal stays in focus): 'DISPIM MODULE +', the diSPIM module has been moved 12 μm above the optimal position; 'DISPIM MODULE -', the diSPIM module has been moved 10 μm below the optimal position; 'Z GALVO +', arm B's Z Galvo has been positioned 0.08 V (sheet has moved 6.5 μm) above the optimal value; 'Z GALVO -', arm B's Z Galvo has been positioned 0.1 V (sheet has moved 8 μm) below the optimal value; 'OBJECTIVE PIEZO +', arm A's objective piezo has been positioned 1 V (plane of detection has moved 20 μm) above the optimal value; 'OBJECTIVE PIEZO -', arm A's objective piezo has been positioned 1 V (plane of detection has moved 20 μm) below the optimal value. The sample is a coverslip coated with yellow-green fluorescent beads. Scalebars: 50 μm.
Supplementary Figure 7 Synchronizing the light sheet and collection objective when performing volumetric imaging.
Schematic (left) and maximum intensity projections of fluorescent beads on a coverslip (right) exemplifying cases in which the light sheet lags behind the collection objective (a), the light sheet and collection objective are well synchronized (b), in which the light sheet moves ahead of collection objective (c). Scalebars: 10 μm.
Supplementary Figure 8 Example showing the effect of varying the Z-Galvo conversion factor.
a. An example of a maximum intensity projection from an imaging volume of a fluorescent bead layer when the Z-galvo conversion factor is optimal and b. when the Z-galvo conversion factor is offset by 0.002V. Scalebars: 10 μm.
Supplementary Figure 9 Examples showing partial and full coverage of an embryo in plane and volume.
a. Shows maximum intensity projections of an embryo which is partially covered (left and middle) and fully covered (right) in X-Y plane. The Y-galvo offset can be changed to bring a partially-covered embryo into full coverage, as shown on the right. b. Maximum-intensity projection of a volume resliced in Z, showing partial (left) and full (right) coverage of an embryo in the Z-direction. If the embryo is only partially-covered in Z, move the diSPIM module until the embryo is fully covered.
Supplementary Figure 10 Maximum intensity projections of nuclei in C. elegans embryos, corresponding to arm A and arm B views.
a. YZ projection captured in arm A. b. YZ projection captured in arm B. c. XY projection captured in arm A and d. XY projection corresponding to arm B. See also Supplementary Video 4.
Supplementary information
Supplementary Text and Figures
Supplementary Notes 1–3 and Supplementary Tables 1–4 (PDF 5023 kb)
Supplementary Figure 1
Diagnostics for assessing excitation input to diSPIM. (PDF 184 kb)
Supplementary Figure 2
Offset and aligned SPIM beams as viewed in the bottom camera. (PDF 221 kb)
Supplementary Figure 3
'Pencil' and epi beams as viewed in SPIM camera. (PDF 183 kb)
Supplementary Figure 4
Fine adjustment of light sheets, as viewed in the bottom camera. (PDF 130 kb)
Supplementary Figure 5
Epifluorescence, SPIM, and overlapped beams as viewed in arm A camera. (PDF 69 kb)
Supplementary Figure 6
Misalignment in diSPIM components diagnosed through arm A camera. (PDF 246 kb)
Supplementary Figure 7
Synchronizing the light sheet and collection objective when performing volumetric imaging. (PDF 154 kb)
Supplementary Figure 8
Example showing the effect of varying the Z-Galvo conversion factor. (PDF 134 kb)
Supplementary Figure 9
Examples showing partial and full coverage of an embryo in plane and volume. (PDF 144 kb)
Supplementary Figure 10
Maximum intensity projections of nuclei in C. elegans embryos, corresponding to arm A and arm B views. (PDF 174 kb)
Volumetric time series of C. elegans embryo (strain BV24) expressing GFP-histones.
C. elegans strain BV24 (ltIs44 [pie-1p-mCherry::PH(PLC1delta1) + unc-119(+)]; zuIs178 [(his-72 1kb::HIS-72::GFP); unc-119(+)] V embryo expressing a GFP-histone fusion protein was imaged for ∼14 hours, demonstrating long-term imaging capabilities of the diSPIM system. Individual frames of the movie were normalized using the 'enhance contrast' command in ImageJ to maintain a similar brightness level throughout the movie. Volumes were sampled every minute, using 45 planes/volume/view and 1 μm inter-plane spacing. Data were derived from arm A and arm B; the presented data has been deconvolved after registration. See also Fig. 3. (AVI 14552 kb)
Volumetric time series of human lung fibroblast cell expressing GFP tagged H-Ras.
DiSPIM image sequence of a cultured human lung fibroblast cell, expressing GFP-tagged H-Ras, and demonstrating dynamic cellular movements. The image stack was converted to 8-bit format in ImageJ, and the 'Hot Red' LUT was applied to it. Volumes were sampled every 5 seconds, at 120 planes/volume/view and 0.5 μm inter-plane spacing. Data were derived from arm A and arm B; the presented data has been deconvolved after registration. See also Fig. 4. (AVI 8684 kb)
Examples of a partially and a fully captured embryo in volumetric imaging.
Examples of a volume where the C. elegans embryo is not completely captured by the z-stack (left images) and where the embryo is completely captured (right images). The volume was sampled at 44 planes/volume and 1 μm inter-plane spacing. The presented data were derived from arm A. See also Supplementary Fig. 9. (AVI 602 kb)
Volumetric images of an embryo collected from arms A and B.
Data derived from arm A (left) and arm B (right) of a C. elegans embryo. The volume was sampled at 40 planes/volume and 1 μm inter-plane spacing. See also Supplementary Fig. 10. (AVI 990 kb)
Supplementary Data 1
Processed (16 bit) C. elegans embryo data using joint deconvolution showing an example of a successful and failed registration. Images of an embryo after registering the images from arm A and B and processing them using joint deconvolution (10 iterations were used in this dataset), showing the effects of successful (left) and failed (right) registration. In the failed registration case, some cells appear displaced or artificially divided (see also Supplementary Note 1 SF1). (ZIP 13231 kb)
Supplementary Data 2
Bead (16 bit) data when the light sheet is synchronized with, lagging, or leading the collection objective: A fluorescent bead layer was imaged in arm A. The volume was sampled at 45 planes/volume and 1 μm inter-plane spacing. synch.tif, demonstrates optimal synchronization between light sheet and the collection objective. For examples that illustrate the effects of light sheet lagging or leading the objective, see lagging.tif, or leading.tif. See also Supplementary Figure 7. (ZIP 5518 kb)
Supplementary Data 3
Bead (16 bit) data acquired by keeping the light sheet stationary and scanning the collection objective. In this example, the light sheet was kept fixed and the collection objective was scanned through the bead layer. The volume was sampled at 99 planes/volume and 0.5 μm inter-plane spacing. This figure shows how best to pick the objective piezo offset. See also step 79. (ZIP 5033 kb)
Supplementary Data 4
Bead (16 bit) data showing the effect of a non-optimal Z-galvo conversion factor. Fluorescent bead data showing the effect of a poor choice of Z-galvo conversion factor, lower by 0.002 V from the optimal value (compare to Supplementary Data 2 (synch.tif)). The lightsheet is ahead of the collection objective initially and lags behind towards the end of the acquisition. The resulting maximum intensity projection shows focused beads only in the middle of the stack, and blurry beads on both ends (see Supplementary Fig. 8.). The volume was sampled at 45 planes/volume and 1 μm inter-plane spacing. (ZIP 2073 kb)
Supplementary Data 5
Bead dataset showing the resolution expected in unregistered/undeconvolved images from individual SPIM arms, and in a registered, deconvolved volume. Images include: (SPIMA.tif) Bead (16 bit) data acquired by SPIMA: Raw data of a fluorescent bead layer, collected in arm A. The volume was sampled at 70 planes/volume and 0.5 μm inter-plane spacing. Also see Fig. 3. (SPIMB.tif) Bead (16 bit) data acquired by SPIMB: Raw data of a fluorescent bead layer, collected in arm B. The volume was sampled at 70 planes/volume and 0.5 μm inter-plane spacing. Also see Fig. 2. The sample is identical to that in Supplementary Data 5 (SPIMA.tif). (deconv.tif) Processed (16 bit) bead data using joint deconvolution: The bead data in Supplementary Data 5 (SPIMA.tif and SPIMB.tif), after registering the datasets and processing them using joint deconvolution (15 iterations were used in this dataset). Also see Fig. 2. (ZIP 4520 kb)
Supplementary Data 6
Bead data (16 bit) acquired by scanning the light sheet and keeping the collection objective fixed at a single plane. An example of data used for measuring the light sheet thickness. The volume was sampled at 99 planes/volume and 0.5 μm inter-plane spacing. See also Fig. 2 and step 95. (ZIP 4838 kb)
Supplementary Data 7
C. elegans embryo dataset, showing the resolution expected in unregistered/undeconvolved images from individual SPIM arms, and in a registered, deconvolved volume. Images include: (SPIMA.tif) Raw (16 bit) data of a C. elegans embryo from arm A: The volume was sampled at 40 planes/volume and 1 μm inter-plane spacing. (SPIMB.tif) Raw (16 bit) data of a C. elegans embryo from arm B: The volume was sampled at 40 planes/volume and 1 μm inter-plane spacing. (deconv.tif) Processed (16 bit) C. elegans embryo data using joint deconvolution: Images of embryo in Supplementary Data 7 (SPIMA.tif and SPIMB.tif) after registering the images and processing them using joint deconvolution (10 iterations were used in this dataset). (ZIP 11833 kb)
Supplementary Data 8
Fibroblast dataset, showing the resolution expected in unregistered/undeconvolved images from individual SPIM arms, and in a registered, deconvolved volume. Images include: (SPIMA.tif) Raw (16 bit) data of a cell from arm A: An image sequence of a cultured human lung fibroblast cell acquired in arm A, expressing GFP-tagged H-Ras. The volume was sampled at 120 planes/volume/view and 0.5 μm inter-plane spacing. (SPIMB.tif) Raw (16 bit) data of a cell from arm B: An acquired image sequence of a cultured human lung fibroblast cell from arm B, expressing GFP-tagged H-Ras. The volume was sampled at 120 planes/volume/view and 0.5 μm inter-plane spacing. (deconv.tif) Processed (16 bit) data of cell using joint deconvolution: Images of a cultured human lung fibroblast cell (SPIMA.tif and SPIMB.tif) after registering the images and processing them using joint deconvolution (10 iterations were used in this dataset). (ZIP 41223 kb)
Rights and permissions
About this article
Cite this article
Kumar, A., Wu, Y., Christensen, R. et al. Dual-view plane illumination microscopy for rapid and spatially isotropic imaging. Nat Protoc 9, 2555–2573 (2014). https://doi.org/10.1038/nprot.2014.172
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.172
This article is cited by
-
Smart lattice light-sheet microscopy for imaging rare and complex cellular events
Nature Methods (2024)
-
Experimental and theoretical model for the origin of coiling of cellular protrusions around fibers
Nature Communications (2023)
-
Fiber enhancement and 3D orientation analysis in label-free two-photon fluorescence microscopy
Scientific Reports (2023)
-
Efficient 3D light-sheet imaging of very large-scale optically cleared human brain and prostate tissue samples
Communications Biology (2023)
-
High activity and high functional connectivity are mutually exclusive in resting state zebrafish and human brains
BMC Biology (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.