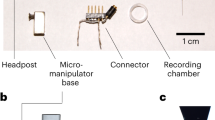
Abstract
It is well established that neural circuits consist of a great diversity of cell types, but very little is known about how neuronal diversity contributes to cognition and behavior. One approach to addressing this problem is to directly link cellular diversity to neuronal activity recorded in vivo in behaving animals. Here we describe the technical procedures for obtaining juxtacellular recordings from single neurons in trained rats engaged in exploratory behavior. The recorded neurons can be labeled to allow subsequent anatomical identification. In its current format, the protocol can be used for resolving the cellular identity of spatially modulated neurons (i.e., head-direction cells and grid cells), which form the basis of the animal's internal representation of space, but this approach can easily be extended to other unrestrained behaviors. The procedures described here, from the beginning of animal training to the histological processing of brain sections, can be completed in ∼3–4 weeks.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Klausberger, T. & Somogyi, P. Neuronal diversity and temporal dynamics: the unity of hippocampal circuit operations. Science 321, 53–57 (2008).
Krook-Magnuson, E., Varga, C., Lee, S.H. & Soltesz, I. New dimensions of interneuronal specialization unmasked by principal cell heterogeneity. Trends Neurosci. 35, 175–184 (2012).
Klausberger, T. et al. Brain-state– and cell-type–specific firing of hippocampal interneurons in vivo. Nature 421, 844–848 (2003).
Ascoli, G.A. et al. Petilla terminology: nomenclature of features of GABAergic interneurons of the cerebral cortex. Nat. Rev. Neurosci. 9, 557–568 (2008).
Lee, W.C.A. & Reid, R.C. Specificity and randomness: structure-function relationships in neural circuits. Curr. Opin. Neurobiol. 21, 801–807 (2011).
Harris, K.D. & Mrsic-Flogel, T.D. Cortical connectivity and sensory coding. Nature 503, 51–8 (2013).
Varga, C., Lee, S.Y. & Soltesz, I. Target-selective GABAergic control of entorhinal cortex output. Nat. Neurosci. 13, 822–824 (2010).
Ray, S. et al. Grid-layout and theta-modulation of layer 2 pyramidal neurons in medial entorhinal cortex. Science 343, 891–896 (2014).
Kerr, J.N.D. et al. Spatial organization of neuronal population responses in layer 2/3 of rat barrel cortex. J. Neurosci. 27, 13316–13328 (2007).
de Kock, C.P.J. & Sakmann, B. Spiking in primary somatosensory cortex during natural whisking in awake head-restrained rats is cell-type specific. Proc. Natl. Acad. Sci. USA 106, 16446–16450 (2009).
Dong, H.-W., Swanson, L.W., Chen, L., Fanselow, M.S. & Toga, A.W. Genomic-anatomic evidence for distinct functional domains in hippocampal field CA1. Proc. Natl. Acad. Sci. USA 106, 11794–11799 (2009).
Thompson, C.L. et al. Genomic anatomy of the hippocampus. Neuron 60, 1010–1021 (2008).
Kamme, F. et al. Single-cell microarray analysis in hippocampus CA1: demonstration and validation of cellular heterogeneity. J. Neurosci. 23, 3607–3615 (2003).
Oberlaender, M. et al. Three-dimensional axon morphologies of individual layer 5 neurons indicate cell type-specific intracortical pathways for whisker motion and touch. Proc. Natl. Acad. Sci. USA 108, 4188–4193 (2011).
Fenno, L., Yizhar, O. & Deisseroth, K. The development and application of optogenetics. Annu. Rev. Neurosci. 34, 389–412 (2011).
Zhang, F. et al. Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat. Protoc. 5, 439–456 (2010).
Deisseroth, K. & Schnitzer, M.J. Engineering approaches to illuminating brain structure and dynamics. Neuron 80, 568–577 (2013).
Wang, J. et al. Integrated device for combined optical neuromodulation and electrical recording for chronic in vivo applications. J. Neural Eng. 9, 016001 (2012).
Anikeeva, P. et al. Optetrode: a multichannel readout for optogenetic control in freely moving mice. Nat. Neurosci. 15, 163–170 (2011).
Prakash, R. et al. Two-photon optogenetic toolbox for fast inhibition, excitation and bistable modulation. Nat. Methods 9, 1171–1179 (2012).
Zhang, F. et al. Multimodal fast optical interrogation of neural circuitry. Nature 446, 633–639 (2007).
Aravanis, A.M. et al. An optical neural interface: in vivo control of rodent motor cortex with integrated fiber-optic and optogenetic technology. J. Neural Eng. 4, S143–S156 (2007).
Lee, J.H. et al. Global and local fMRI signals driven by neurons defined optogenetically by type and wiring. Nature 465, 788–792 (2010).
Cardin, J.A. et al. Targeted optogenetic stimulation and recording of neurons in vivo using cell-type-specific expression of channelrhodopsin-2. Nat. Protoc. 5, 247–254 (2010).
Chung, K. et al. Structural and molecular interrogation of intact biological systems. Nature 497, 332–337 (2013).
Kepecs, A. & Fishell, G. Interneuron cell types are fit to function. Nature 505, 318–326 (2014).
Battaglia, D., Karagiannis, A., Gallopin, T., Gutch, H.W. & Cauli, B. Beyond the frontiers of neuronal types. Front. Neural Circuits 7, 13 (2013).
Denk, W., Briggman, K.L. & Helmstaedter, M. Structural neurobiology: missing link to a mechanistic understanding of neural computation. Nat. Rev. Neurosci. 13, 351–358 (2012).
Lang, S., Dercksen, V.J., Sakmann, B. & Oberlaender, M. Simulation of signal flow in 3D reconstructions of an anatomically realistic neural network in rat vibrissal cortex. Neural Netw. 24, 998–1011 (2011).
Potjans, T.C. & Diesmann, M. The cell-type specific cortical microcircuit: relating structure and activity in a full-scale spiking network model. Cereb. Cortex 24, 785–806 (2014).
Brown, S.P. & Hestrin, S. Cell-type identity: a key to unlocking the function of neocortical circuits. Curr. Opin. Neurobiol. 19, 415–421 (2009).
Pinault, D. Golgi-like labeling of a single neuron recorded extracellularly. Neurosci. Lett. 170, 255–260 (1994).
Deschênes, M., Bourassa, J. & Pinault, D. Corticothalamic projections from layer V cells in rat are collaterals of long-range corticofugal axons. Brain Res. 664, 215–219 (1994).
Pinault, D. A novel single-cell staining procedure performed in vivo under electrophysiological control: morpho-functional features of juxtacellularly labeled thalamic cells and other central neurons with biocytin or Neurobiotin. J. Neurosci. Methods 65, 113–136 (1996).
Pinault, D. The juxtacellular recording-labeling technique. In Electrophysiol. Rec. Tech. Neuromethods (eds. Vertes, R.P. & Stackman, R.W. Jr.) 54, 41–75 (Humana Press, 2011).
Pinault, D., Bourassa, J. & Deschenes, M. The axonal arborization of single thalamic reticular neurons in the somatosensory thalamus of the rat. Eur. J. Neurosci. 7, 31–40 (1995).
Houweling, A. & Brecht, M. Behavioral report of single neurons stimulation in somatosensory cortex. Nature 451, 65–69 (2008).
Mileykovskiy, B. & Morales, M. Duration of inhibition of ventral tegmental area dopamine neurons encodes a level of conditioned fear. J. Neurosci. 31, 7471–7476 (2011).
Boucetta, S., Cisse, Y., Mainville, L., Morales, M. & Jones, B.E. Discharge profiles across the sleep-waking cycle of identified cholinergic, GABAergic, and glutamatergic neurons in the pontomesencephalic tegmentum of the rat. J. Neurosci. 34, 4708–4727 (2014).
Pinault, D. & Deschênes, M. Projection and innervation patterns of individual thalamic reticular axons in the thalamus of the adult rat: a three-dimensional, graphic, and morphometric analysis. J. Comp. Neurol. 391, 180–203 (1998).
Burgalossi, A. et al. Microcircuits of functionally identified neurons in the rat medial entorhinal cortex. Neuron 70, 773–786 (2011).
Herfst, L. et al. Friction-based stabilization of juxtacellular recordings in freely moving rats. J. Neurophysiol. 108, 697–707 (2012).
Lee, A.K., Epsztein, J. & Brecht, M. Head-anchored whole-cell recordings in freely moving rats. Nat. Protoc. 4, 385–392 (2009).
Judkewitz, B., Rizzi, M., Kitamura, K. & Häusser, M. Targeted single-cell electroporation of mammalian neurons in vivo. Nat. Protoc. 4, 862–869 (2009).
Veenman, C.L., Reiner, A. & Honig, M.G. Biotinylated dextran amine as an anterograde tracer for single- and double-labeling studies. J. Neurosci. Methods 41, 239–254 (1992).
Furuta, T., Kaneko, T. & Deschênes, M. Septal neurons in barrel cortex derive their receptive field input from the lemniscal pathway. J. Neurosci. 29, 4089–4095 (2009).
Domnisoru, C., Kinkhabwala, A.A. & Tank, D.W. Membrane potential dynamics of grid cells. Nature 495, 199–204 (2013).
Schwarz, C. et al. The head-fixed behaving rat—procedures and pitfalls. Somatosens. Mot. Res. 27, 131–148 (2010).
Muller, R.U., Kubie, J.L. & Ranck, J.B. Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J. Neurosci. 7, 1935–1950 (1987).
Paxinos, G. & Watson, C. The Rat Brain in Stereotaxic Coordinates (Elsevier Science, 2006).
Lee, A.K., Manns, I.D., Sakmann, B. & Brecht, M. Whole-cell recordings in freely moving rats. Neuron 51, 399–407 (2006).
Mizuseki, K., Sirota, A., Pastalkova, E. & Buzsáki, G. Theta oscillations provide temporal windows for local circuit computation in the entorhinal-hippocampal loop. Neuron 64, 267–280 (2009).
Horikawa, K. & Armstrong, W.E. A versatile means of intracellular labeling: injection of biocytin and its detection with avidin conjugates. J. Neurosci. Methods 25, 1–11 (1988).
Huang, Q., Zhou, D. & DiFiglia, M. Neurobiotin, a useful neuroanatomical tracer for in vivo anterograde, retrograde and transneuronal tract-tracing and for in vitro labeling of neurons. J. Neurosci. Methods 41, 31–43 (1992).
Marx, M., Günter, R.H., Hucko, W., Radnikow, G. & Feldmeyer, D. Improved biocytin labeling and neuronal 3D reconstruction. Nat. Protoc. 7, 394–407 (2012).
Brecht, M. et al. An isomorphic mapping hypothesis of the grid representation. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 369, 20120521 (2014).
Hafting, T., Fyhn, M., Molden, S., Moser, M.-B. & Moser, E.I. Microstructure of a spatial map in the entorhinal cortex. Nature 436, 801–806 (2005).
Taube, J.S., Muller, R.U. & Ranck, J.B. Head-direction cells recorded from the postsubiculum in freely moving rats. I. Description and quantitative analysis. J. Neurosci. 10, 420–435 (1990).
Taube, J.S. The head direction signal: origins and sensory-motor integration. Annu. Rev. Neurosci. 30, 181–207 (2007).
Moser, E.I., Kropff, E. & Moser, M.-B. Place cells, grid cells, and the brain's spatial representation system. Annu. Rev. Neurosci. 31, 69–89 (2008).
Moser, E.I. & Moser, M.-B. Grid cells and neural coding in high-end cortices. Neuron 80, 765–774 (2013).
Canto, C.B. & Witter, M.P. Cellular properties of principal neurons in the rat entorhinal cortex. II. The medial entorhinal cortex. Hippocampus 22, 1277–1299 (2012).
Burgalossi, A. & Brecht, M. Cellular, columnar and modular organization of spatial representations in medial entorhinal cortex. Curr. Opin. Neurobiol. 24, 47–54 (2014).
Sargolini, F. et al. Conjunctive representation of position, direction, and velocity in entorhinal cortex. Science 312, 758–762 (2006).
Boccara, C.N. et al. Grid cells in pre- and parasubiculum. Nat. Neurosci. 13, 987–994 (2010).
Acknowledgements
We thank A. Stern and M. Kunert (Berlin, Germany) and K. Vollmer (Tübingen, Germany) for excellent fine mechanical support, and U. Schneeweiß and J. Steger for excellent technical support. We are particularly grateful to R. Naumann and S. Ray for histological processing of the cells shown in Figures 4 and 5, U. Schneeweiß for reconstructing the neuron shown in Figure 4a and C. Mende for contributions to figures. We thank G. Doron for comments on earlier versions of the manuscript. This work was supported by Neurocure, the Bernstein Center for Computational Neuroscience (BMBF) and Humboldt University, an EU Biotact-grant and a Neuro-behavior European Research Council grant.
Author information
Authors and Affiliations
Contributions
A.B. and Q.T. performed experiments in the establishment of the protocol. A.B. and M.B. supervised the experiments. A.B. drafted the manuscript. Q.T., M.B. and A.B. contributed to, and have approved, the final version of the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Tang, Q., Brecht, M. & Burgalossi, A. Juxtacellular recording and morphological identification of single neurons in freely moving rats. Nat Protoc 9, 2369–2381 (2014). https://doi.org/10.1038/nprot.2014.161
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.161
This article is cited by
-
Linking neuronal structure to function in rodent hippocampus: a methodological prospective
Cell and Tissue Research (2018)
-
Vibrissa motor cortex activity suppresses contralateral whisking behavior
Nature Neuroscience (2017)
-
The enigmatic mossy cell of the dentate gyrus
Nature Reviews Neuroscience (2016)
-
An optogenetics- and imaging-assisted simultaneous multiple patch-clamp recording system for decoding complex neural circuits
Nature Protocols (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.