Abstract
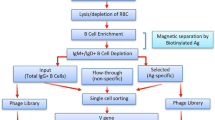
This protocol describes the design and development of recombinant monovalent antigen-binding molecules derived from monoclonal antibodies through rapid identification and cloning of the functional variable heavy (VH) and variable light (VL) genes and the design and cloning of a synthetic DNA sequence optimized for expression in recombinant bacteria. Typically, monoclonal antibodies are obtained from mouse hybridomas, which most often result from the fusion of B lymphocytes from immunized mice with murine myeloma cells. The protocol described here has previously been exploited for the successful development of multiple antibody-based molecules targeting a wide range of biomolecular targets. The protocol is accessible for research groups who may not be specialized in this area, and should permit the straightforward reverse engineering of functional, recombinant antigen-binding molecules from hybridoma cells secreting functional IgGs within 50 working days. Furthermore, convenient strategies for purification of antibody fragments are described.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Köhler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Nelson, A.L. & Reichert, J.M. Development trends for therapeutic antibody fragments. Nat. Biotechnol. 27, 331–337 (2009).
Bird, R.E. et al. Single-chain antigen-binding proteins. Science 242, 423–426 (1988).
Holliger, P. & Hudson, P.J. Engineered antibody fragments and the rise of single domains. Nat. Biotechnol. 23, 1126–1136 (2005).
Aubrey, N. et al. Engineering of a recombinant Fab from a neutralizing IgG directed against scorpion neurotoxin AahI and functional evaluation versus other antibody fragments. Toxicon 43, 233–241 (2004).
Eisenhardt, S.U., Schwarz, M., Bassler, N. & Peter, K. Subtractive single-chain antibody (scFv) phage-display: tailoring phage-display for high specificity against function-specific conformations of cell membrane molecules. Nat. Protoc. 2, 3063–3073 (2007).
Chapman, A.P. PEGylated antibodies and antibody fragments for improved therapy: a review. Adv. Drug Deliv. Rev. 54, 531–545 (2002).
Zahid, M. et al. Design and reshaping of an scFv directed against human platelet glycoprotein VI with diagnostic potential. Anal. Biochem. 417, 274–282 (2011).
Dubreuil, O. et al. Fine tuning of the specificity of an anti-progesterone antibody by first and second sphere residue engineering. J. Biol. Chem. 280, 24880–24887 (2005).
Rouet, R. et al. Expression of high-affinity human antibody fragments in bacteria. Nat. Protoc. 7, 364–373 (2012).
Muzard, J. et al. Design and humanization of a murine scFv that blocks human platelet glycoprotein VI in vitro. FEBS J. 276, 4207–4222 (2009).
di Tommaso, A. et al. Diabody mixture providing full protection against experimental scorpion envenoming with crude Androctonus australis venom. J. Biol. Chem. 287, 14149–14156 (2012).
Kontermann, R.E. Strategies to extend plasma half-lives of recombinant antibodies. Biodrugs 23, 93–109 (2009).
Juste, M., Martin-Eauclaire, M.F., Devaux, C., Billiald, P. & Aubrey, N. Using a recombinant bispecific antibody to block Na+-channel toxins protects against experimental scorpion envenoming. Cell Mol. Life Sci. 64, 206–218 (2007).
Aubrey, N. et al. Design and evaluation of a diabody to improve protection against a potent scorpion neurotoxin. Cell Mol. Life Sci. 60, 617–628 (2003).
Nelson, A.L., Dhimolea, E. & Reichert, J.M. Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 9, 767–774 (2010).
Barbas III, C.F., Burton, D.R., Scott, J.K. & Silverman, G.J. Phage Display: A laboratory Manual (Cold Spring Harbor Laboratory Press, 2004).
Grimm, S., Yu, F. & Nygren, P.A. Ribosome display selection of a murine IgG(1) Fab binding affibody molecule allowing species selective recovery of monoclonal antibodies. Mol. Biotechnonol. 48, 263–276 (2011).
Chao, G. et al. Isolating and engineering human antibodies using yeast surface display. Nat. Protoc. 1, 755–768 (2006).
Cotten, S.W., Zou, J., Valencia, C.A. & Liu, R. Selection of proteins with desired properties from natural proteome libraries using mRNA display. Nat. Protoc. 6, 1163–1182 (2011).
Mazor, Y., Blarcom, T.V., Iverson, B.L. & Georgiou, G. E-clonal antibodies: selection of full-length IgG antibodies using bacterial periplasmic display. Nat. Protoc. 3, 1766–1777 (2008).
Haque, A. & Tonks, K.T. The use of phage display to generate conformation-sensor recombinant antibodies. Nat. Protoc. 7, 2127–2143 (2012).
Lee, C.M.Y., Iorno, N., Sierro, F. & Christ, D. Selection of human antibody fragments by phage display. Nat. Protoc. 2, 3001–3008 (2007).
O'Connell, D., Becerril, B., Roy-Burman, A., Daws, M. & Marks, J.D. Phage versus phagemid libraries for generation of human monoclonal antibodies. J. Mol. Biol. 321, 49–56 (2002).
Bourbeillon, J. et al. Minimum information about a protein affinity reagent (MIAPAR). Nat. Biotechnol. 28, 650–653 (2010).
Borrebaeck, C.A.K. Antibody Engineering, 2nd edn. (Oxford University Press, 1994).
Gustafsson, C., Govindarajan, S. & Minshull, J. Codon bias and heterologous protein expression. Trends Biotechnol. 22, 346–353 (2004).
Sambrook, J., Fritsch, E.F. & Maniatis, T. Molecular Cloning: a Laboratory Manual 3rd edn. (eds. Sambrook, J. & Russell, D.W.) 11.98–11.133 (Cold Spring Harbor Laboratory Press, 2001).
Muzard, J., Loyau, S., Ajzenberg, N., Billiald, P. & Jandrot-Perrus, M. Antithrombotic recombinant antibodies. J. Soc. Biol. 200, 365–376 (2006).
McGregor, D.P., Molloy, P.E., Cunningham, C. & Harris, W.J. Spontaneous assembly of bivalent single chain antibody fragments in Escherichia coli. Mol. Immunol. 31, 219–226 (1994).
Muzard, J. Recent patent applications in antibody fragments: an academic update from the EU. Nat. Biotechnol. 29, 979 (2011).
Muzard, J., Fields, C., O'Mahony, J.J. & Lee, G.U. Probing the soybean Bowman Birk Inhibitor using recombinant antibody fragments. J. Agric. Food Chem. 60, 6164–6172 (2012).
Kortt, A.A., Dolezal, O., Power, B.E. & Hudson, P.J. Dimeric and trimeric antibodies: high avidity scFvs for cancer targeting. Biomol. Eng. 18, 95–108 (2001).
Muzard, J. et al. Grafting of protein L-binding activity onto recombinant antibody fragments. Anal Biochem. 388, 331–338 (2009).
Juste, M., Muzard, J. & Billiald, P. Cloning of the antibody κ light chain V-gene from murine hybridomas by bypassing the aberrant MOPC21-derived transcript. Anal Biochem. 349, 159–161 (2006).
Heo, M.A. et al. Functional expression of single-chain variable fragment antibody against c-Met in the cytoplasm of Escherichia coli. Protein Expr. Purif. 47, 203–209 (2006).
Tolner, B., Smith, L., Begent, R.H.J. & Chester, K.A. Production of recombinant protein in Pichia pastoris by fermentation. Nat. Protoc. 1, 1006–1021 (2006).
Hardiman, G. Next-generation antibody discovery platforms. Proc. Natl Acad. Sci. USA 109, 1824–1826 (2012).
Kabat, E.A., Wu, T.T., Perry, H., Gottesman, G. & Foeller, C. Sequences of Proteins of Immunological Interest 5th edn. US Department of Health and Human Services, Public Health Service, National Institutes of Health (NIH): NIH publication no. 91-3242 (1991).
Hornig, N. & Färber-Schwarz, A. Production of bispecific antibodies: diabodies and tandem scFv. Methods Mol. Biol. 907, 713–727 (2012).
Ruberti, F., Cattaneo, A. & Bradbury, A. The use of the RACE method to clone hybridoma cDNA when V region primers fail. J. Immunol. Methods 173, 33–39 (1994).
Kaas, Q. & Lefranc, M.P. IMGT Colliers de Perles: standardized sequence-structure representations of the IgSF and MhcSF superfamily domains. Curr. Bioinformatics 2, 21–30 (2007).
Kaas, Q., Ehrenmanns, F. & Lefranc, M.P. IG, TR, MHC, IgSf and MhcSF: what do we learn from the IMGT Colliers de Perles? Brief. Funct. Genomic Proteomic. 6, 253–264 (2007).
Honegger, A. & Plückthun, A. Yet another numbering scheme for immunoglobulin variable domains: An automatic modeling and analysis tool. J. Mol. Biol. 309, 657–670 (2001).
Billiald, P., Motta, G. & Vaux, D.J. Production of a functional anti-scorpion hemocyanin scFv in Escherichia coli. Arch. Biochem. Biophys. 317, 429–438 (1995).
Zhou, H., Fisher, R.J. & Papas, T.S. Optimization of primer sequences for mouse scFv repertoire display library construction. Nucleic Acids Res. 22, 888–889 (1994).
Ortega, C. et al. High level prokaryotic expression of anti-Müllerian inhibiting substance type II receptor diabody, a new recombinant antibody for in vivo ovarian cancer imaging. J. Immunol. Methods 387, 11–20 (2013).
Schaefer, J.V. & Plückthun, A. Improving expression of scFv fragments by co-expression of periplasmic chaperones. in Antibody Engineering 2nd edn. (eds. Kontermann, R. & Dübel, S.) 2, 345–361 (Springer, 2010).
Vincze, T., Posfai, J. & Roberts, R.J. NEBcutter: a program to cleave DNA with restriction enzymes. Nucleic Acids Res. 31, 3688–3691 (2003).
Ehrenmann, F., Giudicelli, V., Duroux, P. & Lefranc, M.P. IMGT/Collier de Perles: IMGT standardized representation of domains (IG, TR, and IgSF variable and constant domains, MH and MhSF groove domains). Cold Spring Harbor Protoc. 6, 726–736 (2011).
Martin, A.C.R. Accessing the Kabat antibody sequence database by computer PROTEINS: Structure, Function and Genetics. Proteins 25, 130–133 (1996).
Whitelegg, N.R.J. & Rees, A.R. WAM: an improved algorithm for modeling antibodies on the web. Protein Eng. 13, 819–824 (2000).
Bruccoleri, R.E. Application of systematic conformational search to protein modelling. Mol. Simul. 10, 151–174 (1993).
Sivasubramanian, A., Sircar, A., Chaudhury, S. & Gray, J.J. Toward high-resolution homology modeling of antibody Fv regions and application to antibody-antigen docking. Proteins 74, 497–514 (2009).
Duhovny, D., Nussinov, R. & Wolfson, H.J. Efficient unbound docking of rigid molecules. in Proceedings of the 2nd Workshop on Algorithms in Bioinformatics (WABI), Rome, Italy; Lecture Notes in Computer Science (ed. Gusfield et al.) 2452, 185–200 (Springer, 2002).
Schneidman-Duhovny, D., Inbar, Y., Nussinov, R. & Wolfson, H.J. PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res. 33, W363–W367 (2005).
Acknowledgements
C.F., G.U.L. and J.M. were supported by the EU (MagPro2Life Consortium), Science Foundation Ireland (grant no. 08/IN/2972) and University College Dublin. D.O. was supported by Science Foundation Ireland (grant no. SFI12/IP/1260). P.B. was supported by grant no. 07-EMPB-002-01 from the Agence Nationale de la Recherche.
Author information
Authors and Affiliations
Contributions
This manuscript is based on the protocols developed in our research laboratories from 2002 to 2013. All authors contributed to writing the manuscript and approved its final version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Figure 1
Annotated Fd chain sequences with native leader sequences, FR and CDR according to IMGT (continued line) and Kabat (dotted line) numbering schemes. (PDF 733 kb)
Supplementary Figure 2
Annotated light chain sequences with native leader sequences, FR and CDR according to IMGT (continued line) and Kabat (dotted line) numbering schemes. (PDF 719 kb)
Supplementary Figure 3
Example of a 3D Fv homology model generated using [Box 1, OR12-13]. (PDF 981 kb)
Supplementary Figure 4
Example of an epitope-paratope interaction prediction generated using [Box 1, OR12-13]. (PDF 458 kb)
Rights and permissions
About this article
Cite this article
Fields, C., O'Connell, D., Xiao, S. et al. Creation of recombinant antigen-binding molecules derived from hybridomas secreting specific antibodies. Nat Protoc 8, 1125–1148 (2013). https://doi.org/10.1038/nprot.2013.057
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.057
This article is cited by
-
Developing of specific monoclonal recombinant antibody fused to alkaline phosphatase (AP) for one-step detection of fig mosaic virus
3 Biotech (2022)
-
The structure of serum resistance-associated protein and its implications for human African trypanosomiasis
Nature Microbiology (2018)
-
Transient expression of human antibodies in mammalian cells
Nature Protocols (2018)
-
Down regulation of ADAM33 as a Predictive Biomarker of Aggressive Breast Cancer
Scientific Reports (2017)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.