Abstract
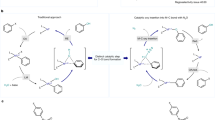
We describe the synthesis of the lesser-known stoichiometric oxidation reagent 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate (1, Bobbitt's salt), as well as of 4-acetamido-(2,2,6,6-tetramethyl-piperidin-1-yl)oxyl (2, AcNH-TEMPO). Several representative oxidation reactions are also presented to demonstrate the salt's oxidative capabilities. Bobbitt's salt has a range of applications, from the oxidation of various alcohols to their corresponding carbonyl derivatives to the oxidative cleavage of benzyl ethers, whereas 2 has been shown to serve as a catalytic or stoichiometric oxidant. The oxyl radical can be obtained in 85% yield over two steps on a 1-mole scale from commercially available 4-amino-2,2,6,6-tetramethylpiperidine (5), and is far more cost-effective to prepare in-house than purchase commercially. An additional step converts the oxyl radical into the oxoammonium salt (1, Bobbitt's salt) in 88% yield, with an overall yield of 75%. The synthesis of the salt takes ∼5 d to complete. Oxoammonium salts are metal-free, nontoxic and environmentally friendly oxidants. Preparation of 1 is also inherently ′green′, as water can be used as the solvent and the use of environmentally unfriendly materials is minimal. Moreover, after it has been used, the spent oxidant can be recovered and used to regenerate 1, thereby making the process recyclable.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Smith, M.B. & March, J. March's Advanced Organic Chemistry, 5th edn. Wiley, 2001.
Bäckvall, J.-E. Modern Oxidation Methods. Wiley-VCH, 2004.
Trost, B.M. & Fleming, I. Comprehensive Organic Synthesis: Selectivity, Strategy, and Efficiency in Modern Organic Chemistry. Oxford: Pergamon Press, 1991.
Caron, S. Practical Synthetic Organic Chemistry: Reactions, Principles, and Techniques. Wiley-VCH, 2011.
Bowden, K., Heilbron, I.M., Jones, E.R.H. & Weedon, B.C.L. Researches on acetylenic compounds. Part I. The preparation of acetylenic ketones by oxidation of acetylenic carbinols and glycols. J. Chem. Soc. 39–45 (1946).
Tojo, G. & Fernández, M. Oxidation of Alcohols to Aldehydes and Ketones. Springer, 2006.
Collins, J.C., Hess, W.W. & Frank, F.J. Dipyridine-chromium(VI) oxide oxidation of alcohols in dichloromethane. Tetrahedron Lett. 9, 3363–3366 (1968).
Corey, E.J. & Suggs, J.W. Pyridinium chlorochromate. An efficient reagent for oxidation of primary and secondary alcohols to carbonyl compounds. Tetrahedron Lett. 16, 2647–2650 (1975).
Corey, E.J. & Schmidt, G. Useful procedures for the oxidation of alcohols involving pyridinium dichromate in aprotic media. Tetrahedron Lett. 20, 399–402 (1979).
Oppenauer, R.V. Eine Methode der Dehydrierung von Sekundären Alkoholen zu Ketonen. I. Zur Herstellung von Sterinketonen und Sexualhormonen. Recl. Trav. Chim. Pays-Bas 56, 137–145 (1937).
Graves, C.R., Campbell, E.J. & Nguyen, S.T. Aluminum-based catalysts for the asymmetric Meerwein–Schmidt–Ponndorf–Verley–Oppenauer (MSPVO) reaction manifold. Tetrahedron: Asymmetry 16, 3460 (2005).
Mandell, L. The mechanism of the Wettstein-Oppenauer oxidation. J. Am. Chem. Soc. 78, 3199–3201 (1956).
Tidwell, T.T. Oxidation of alcohols to carbonyl compounds via alkoxysulfonium ylides: the Moffatt, Swern, and related oxidations. Org. React. 39, 297 (1990).
Mancuso, A.J., Huang, S.-L. & Swern, D. Oxidation of long-chain and related alcohols to carbonyls by dimethyl sulfoxide 'activated' by oxalyl chloride. J. Org. Chem. 43, 2480–2482 (1978).
Corey, E.J. & Kim, C.U. New and highly effective method for the oxidation of primary and secondary alcohols to carbonyl compounds. J. Am. Chem. Soc. 94, 7586–7587 (1972).
Tidwell, T.T. Oxidation of alcohols by activated dimethyl sulfoxide and related reactions: an update. Synthesis 10, 857–870 (1990).
Parikh, J.R. & Doering, W.v.E. Sulfur trioxide in the oxidation of alcohols by dimethyl sulfoxide. J. Am. Chem. Soc. 89, 5505–5507 (1967).
Ley, S.V., Norman, J., Griffith, W.P. & Marsden, S.P. Tetrapropylammonium perruthenate, Pr4N+RuO4−, TPAP: a catalytic oxidant for organic synthesis. Synthesis 7, 639–666 (1994).
Frigerio, M., Santagostino, M. & Sputore, S. A user-friendly entry to 2-iodoxybenzoic acid (IBX). J. Org. Chem. 64, 4537–4538 (1999).
Frigerio, M. & Santagostino, M. A mild oxidizing reagent for alcohols and 1,2-diols: o-Iodoxybenzoic acid (IBX) in DMSO. Tetrahedron Lett. 35, 8019–8022 (1994).
Duschek, A. & Kirsch, S.F. 2-Iodoxybenzoic acid—a simple oxidant with a dazzling array of potential applications. Angew. Chem. Int. Ed. 50, 1524–1552 (2011).
Dess, D.B. & Martin, J.C. Readily accessible 12-I-5 oxidant for the conversion of primary and secondary alcohols to aldehydes and ketones. J. Org. Chem. 48, 4155–4156 (1983).
Tohma, H. & Kita, Y. Hypervalent iodine reagents for the oxidation of alcohols and their application to complex molecule synthesis. Adv. Synth. Catal. 346, 111 (2004).
Cohen, M.D., Kargacin, B., Klein, C.B. & Costa, M. Mechanisms of chromium carcinogenicity and toxicity. Crit. Rev. Toxicol. 23, 255–281 (1993).
Tilley, L.J., Bobbitt, J.M., Murray, S.A., Camire, C.E. & Eddy, N.A. A revised preparation of 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxyl and 4-acetamido-2,2,6,6-tetramethyl-1-oxopiperidinium tetrafluoroborate: reagents for stoichiometric oxidations of alcohols. Synthesis 45, 326–329 (2013).
Bobbitt, J.M. & Flores, C.L. Organic nitrosonium salts as oxidants in organic chemistry. Heterocycles 27, 509–533 (1988).
Shibuya, M., Tomizawa, M. & Iwabuchi, Y. Oxidative rearrangement of tertiary allylic alcohols employing oxoammonium salts. J. Org. Chem. 73, 4750–4752 (2008).
Bobbitt, J.M. Oxoammonium salts. 6. 4-Acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium perchlorate: a stable and convenient reagent for the oxidation of alcohols. Silica gel catalysis. J. Org. Chem. 63, 9367–9374 (1998).
Bobbitt, J.M. & Merbouh, N. Preparation of 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate, and the oxidation of geraniol to geranial. Org. Synth. 82, 80–86 (2005).
Bailey, W.F., Bobbitt, J.M. & Wiberg, K.B. Mechanism of the oxidation of alcohols by oxoammonium cations. J. Org. Chem. 72, 4504–4509 (2007).
Qui, J., Pradhan, P.P., Blanck, N.B., Bobbitt, J.M. & Bailey, W.F. Selective oxoammonium salt oxidations of alcohols to aldehydes and aldehydes to carboxylic acids. Org. Lett. 14, 350–353 (2012).
Bobbitt, J.M., Bruckner, C. & Merbouh, N. Oxoammonium- and nitroxide-catalyzed oxidations of alcohols. Org. React. 74, 103–427 (2010).
Pradhan, P.P., Bobbitt, J.M. & Bailey, W.F. Oxidative cleavage of benzylic and related ethers, using an oxoammonium salt. J. Org. Chem. 74, 9524–9527 (2009).
Merbouh, N., Bobbitt, J.M. & Bruckner, C. Oxoammonium Salts. 9. Oxidative dimerization of polyfunctional primary alcohols to esters. An interesting β oxygen effect. J. Org. Chem. 69, 5116–5119 (2004).
Zakrzewski, J., Grodner, J., Bobbitt, J.M. & Karpiáska, M. Oxidation of unsaturated primary alcohols and ω-haloalkanols with 4-acetylamino-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate. Synthesis 16, 2491–2494 (2007).
Kelly, C.B., Mercadante, M.A., Hamlin, T.A., Fletcher, M.H. & Leadbeater, N.E. Oxidation of α-trifluoromethyl alcohols using a recyclable oxoammonium salt. J. Org. Chem. 77, 8131–8141 (2012).
Shibuya, M., Tomizawa, M., Suzuki, I. & Iwabuchi, Y. 2-Azaadamantane N-Oxyl (AZADO) and 1-Me-AZADO: highly efficient organocatalysts for oxidation of alcohols. J. Am. Chem. Soc. 128, 8412–8413.
Tojo, G. & Fernandez, M.I. Oxidation of Primary Alcohols to Carboxylic Acids: A Guide to Current Common Practice, 1st edn. Springer, 2007.
Sheldon, R.A., Arends, I.W.C.E., ten Brink, G.-J. & Dijksman, A. Green, catalytic oxidations of alcohols. Acc. Chem. Res. 35, 774–781 (2002).
Ciriminna, R. & Pagliaro, M. Industrial oxidations with organocatalyst TEMPO and its derivatives. Org. Process Res. Dev. 14, 245–251 (2010).
Anelli, P.L., Montanari, F. & Quici, S. A general synthetic method for the oxidation of primary alcohols to aldehydes: (S)-(+)-2-Methylbutanal. Org. Synth. 69, 212–219 (1990).
Anelli, P.L., Biffi, C., Montanari, F. & Quici, S. Fast and selective oxidation of primary alcohols to aldehydes or to carboxylic acids and of secondary alcohols to ketones mediated by oxoammonium salts under two-phase conditions. J. Org. Chem. 52, 2559–2562 (1987).
Anelli, P.L., Banfi, S., Montanari, F. & Quici, S. Oxidation of diols with alkali hypochlorites catalyzed by oxammonium salts under two-phase conditions. J. Org. Chem. 54, 2970–2972 (1989).
Ma, Z. & Bobbitt, J.M. Organic oxoammonium salts. 3. A new convenient method for the oxidation of alcohols to aldehydes and ketones. J. Org. Chem. 56, 6110–6614 (1991).
Banwell, M.G., Bridges, V.S., Dupuche, J.R., Richards, S.L. & Walter, J.M. Oxidation of vic-Diols to .alpha.-dicarbonyl compounds using the oxoammonium salt derived from 4-acetamido-TEMPO and p-toluenesulfonic acid. J. Org. Chem. 59, 6338–6343 (1994).
Eddy, N.A., Kelly, C.B., Mercadante, M.A., Leadbeater, N.E. & Fenteany, G. Access to dienophilic ene-triketone synthons by oxidation of diketones with an oxoammonium salt. Org. Lett. 14, 498–501 (2012).
Pradhan, P.P., Bobbitt, J.M. & Bailey, W.F. Ene-like addition of an oxoammonium cation to alkenes: highly selective route to allylic alkoxyamines. Org. Lett. 8, 5485–5487 (2006).
Richter, H. & García-Mancheño, O. Dehydrogenative functionalization of C(sp3)-H bonds adjacent to a heteroatom mediated by oxoammonium salts. Eur. J. Org. Chem. 2010, 4460–4467 (2010).
Farkas, L. & Lewin, M. Analysis of hypochlorite-hypobromite solutions. Anal. Chem. 19, 662–664 (1947).
Adler, N., Litt, G.J. & Johl, R.G. Coulometric titration of hypochlorite ion. Anal. Chem. 39, 226–227 (1967).
Hashmi, M.H., Rashid, A., Ayaz, A.A. & Chughtai, N.A. Indirect determination of hypochlorite and hypobromite by thallium. Anal. Chem. 38, 507–508 (1966).
Richter, H., Rohlmann, R. & García-Mancheño, O. Catalyzed selective direct α- and γ-alkylation of aldehydes with cyclic benzyl ethers by using T+BF−4 in the presence of an inexpensive organic acid or anhydride. Chem. Eur. J. 17, 11622–11627 (2011).
Richter, H. & García-Mancheño, O. TEMPO Oxoammonium salt-mediated dehydrogenative Povarov/oxidation tandem reaction of N-alkyl anilines. Org. Lett. 13, 6066–6069 (2011).
Author information
Authors and Affiliations
Contributions
N.E.L. coordinated the project. C.B.K., M.A.M., J.M.B. and L.J.T. performed the reactions. C.B.K., M.A.M. and N.E.L. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Mercadante, M., Kelly, C., Bobbitt, J. et al. Synthesis of 4-acetamido-2,2,6,6-tetramethylpiperidine-1-oxoammonium tetrafluoroborate and 4-acetamido-(2,2,6,6-tetramethyl-piperidin-1-yl)oxyl and their use in oxidative reactions. Nat Protoc 8, 666–676 (2013). https://doi.org/10.1038/nprot.2013.028
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.028
This article is cited by
-
Oxidative cleavage of C–C bonds in lignin
Nature Chemistry (2021)
-
The “one-pot” synthesis of 2,5-diformylfuran, a promising synthon for organic materials in the conversion of biomass
Russian Chemical Bulletin (2015)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.