Abstract
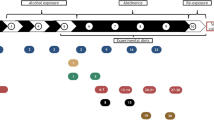
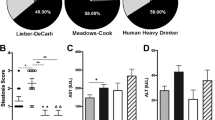
Chronic alcohol consumption is a leading cause of chronic liver disease worldwide, leading to cirrhosis and hepatocellular carcinoma. Currently, the most widely used model for alcoholic liver injury is ad libitum feeding with the Lieber-DeCarli liquid diet containing ethanol for 4–6 weeks; however, this model, without the addition of a secondary insult, only induces mild steatosis, slight elevation of serum alanine transaminase (ALT) and little or no inflammation. Here we describe a simple mouse model of alcoholic liver injury by chronic ethanol feeding (10-d ad libitum oral feeding with the Lieber-DeCarli ethanol liquid diet) plus a single binge ethanol feeding. This protocol for chronic-plus-single-binge ethanol feeding synergistically induces liver injury, inflammation and fatty liver, which mimics acute-on-chronic alcoholic liver injury in patients. This feeding protocol can also be extended to chronic feeding for longer periods of time up to 8 weeks plus single or multiple binges. Chronic-binge ethanol feeding leads to high blood alcohol levels; thus, this simple model will be very useful for the study of alcoholic liver disease (ALD) and of other organs damaged by alcohol consumption.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$259.00 per year
only $21.58 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Gao, B. & Bataller, R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology 141, 1572–1585 (2011).
Tsukamoto, H. & Lu, S.C. Current concepts in the pathogenesis of alcoholic liver injury. FASEB J. 15, 1335–1349 (2001).
O'Shea, R.S., Dasarathy, S. & McCullough, A.J. Practice Guideline Committee of the American Association for the Study of Liver, D. & Practice Parameters Committee of the American College of, G. Alcoholic liver disease. Hepatology 51, 307–328 (2010).
Stickel, F. & Seitz, H.K. Alcoholic steatohepatitis. Best Pract. Res. Clin. Gastroenterol. 24, 683–693 (2010).
Beier, J.I., Arteel, G.E. & McClain, C.J. Advances in alcoholic liver disease. Curr. Gastroenterol. Rep. 13, 56–64 (2011).
Bellentani, S . et al. Drinking habits as cofactors of risk for alcohol induced liver damage. The Dionysos Study Group. Gut 41, 845–850 (1997).
Stranges, S. et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcohol. Clin. Exp. Res. 28, 949–956 (2004).
Li, T.K. Quantifying the risk for alcohol-use and alcohol-attributable health disorders: present findings and future research needs. J. Gastroenterol. Hepatol. 23 (suppl. 1), S2–S8 (2008).
Ki, S.H. et al. Interleukin-22 treatment ameliorates alcoholic liver injury in a murine model of chronic-binge ethanol feeding: role of signal transducer and activator of transcription 3. Hepatology 52, 1291–1300 (2010).
Mathurin, P. & Lucey, M.R. Management of alcoholic hepatitis. J. Hepatol. 56 (suppl. 1), S39–S45 (2012).
Choi, G. & Runyon, B.A. Alcoholic hepatitis: a clinician's guide. Clin. Liver Dis. 16, 371–385 (2012).
Altamirano, J. & Bataller, R. Alcoholic liver disease: pathogenesis and new targets for therapy. Nat. Rev. Gastroenterol. and Hepatol. 8, 491–501 (2011).
Aroor, A.R., Jackson, D.E. & Shukla, S.D. Elevated activation of ERK1 and ERK2 accompany enhanced liver injury following alcohol binge in chronically ethanol-fed rats. Alcohol. Clin. Exp. Res. 35, 2128–2138 (2011).
Petrasek, J. et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J. Clin. Invest. 122, 3476–3489 (2012).
Cohen, J.I., Roychowdhury, S., McMullen, M.R., Stavitsky, A.B. & Nagy, L.E. Complement and alcoholic liver disease: role of C1q in the pathogenesis of ethanol-induced liver injury in mice. Gastroenterology 139, 664–674 (2010).
Mandrekar, P., Ambade, A., Lim, A., Szabo, G. & Catalano, D. An essential role for monocyte chemoattractant protein-1 in alcoholic liver injury: regulation of proinflammatory cytokines and hepatic steatosis in mice. Hepatology 54, 2185–2197 (2011).
Nath, B. et al. Hepatocyte-specific hypoxia-inducible factor-1α is a determinant of lipid accumulation and liver injury in alcohol-induced steatosis in mice. Hepatology 53, 1526–1537 (2011).
Hu, M. et al. Regulation of hepatic lipin-1 by ethanol: role of AMP-activated protein kinase/sterol regulatory element-binding protein 1 signaling in mice. Hepatology 55, 437–446 (2012).
Liangpunsakul, S. et al. Imipramine blocks ethanol-induced ASMase activation, ceramide generation, and PP2A activation, and ameliorates hepatic steatosis in ethanol-fed mice. Am. J. Physiol. Gastrointest. Liver Physiol. 302, G515–523 (2012).
Leung, T.M. et al. Argininosuccinate synthase conditions the response to acute and chronic ethanol-induced liver injury in mice. Hepatology 55, 1596–1609 (2012).
Zhou, Z. et al. Zinc supplementation prevents alcoholic liver injury in mice through attenuation of oxidative stress. Am. J. Pathol. 166, 1681–1690 (2005).
Ueno, A. et al. Mouse intragastric infusion (iG) model. Nat. Protoc. 7, 771–781 (2012).
Xu, J. et al. Synergistic steatohepatitis by moderate obesity and alcohol in mice despite increased adiponectin and p-AMPK. J. Hepatol. 55, 673–682 (2011).
Tsukamoto, H. et al. Severe and progressive steatosis and focal necrosis in rat liver induced by continuous intragastric infusion of ethanol and low fat diet. Hepatology 5, 224–232 (1985).
Kisseleva, T. et al. Myofibroblasts revert to an inactive phenotype during regression of liver fibrosis. Proc. Natl. Acad. Sci. USA 109, 9448–9453 (2012).
Zhou, Z. et al. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-α production. Am. J. Pathol. 163, 1137–1146 (2003).
Zhou, Z., Sun, X. & James Kang, Y. Metallothionein protection against alcoholic liver injury through inhibition of oxidative stress. Exp. Biol. Med. 227, 214–222 (2002).
Beier, J.I., Kaiser, J.P., Guo, L., Martinez-Maldonado, M. & Arteel, G.E. Plasminogen activator inhibitor-1 deficient mice are protected from angiotensin II-induced fibrosis. Arch. Biochem. Biophys. 510, 19–26 (2011).
Kao, E., Shinohara, M., Feng, M., Lau, M.Y. & Ji, C. Human immunodeficiency virus protease inhibitors modulate Ca2+ homeostasis and potentiate alcoholic stress and injury in mice and primary mouse and human hepatocytes. Hepatology 56, 594–604 (2012).
Cook, R.T. et al. Thymocytes, pre-B cells, and organ changes in a mouse model of chronic ethanol ingestion–absence of subset-specific glucocorticoid-induced immune cell loss. Alcohol. Clin. Exp. Res. 31, 1746–1758 (2007).
Meadows, G.G., Blank, S.E. & Duncan, D.D. Influence of ethanol consumption on natural killer cell activity in mice. Alcohol. Clin. Exp. Res. 13, 476–479 (1989).
Coleman, R.A., Young, B.M., Turner, L.E. & Cook, R.T. A practical method of chronic ethanol administration in mice. Methods Mol. Biol. 447, 49–59 (2008).
Brandon-Warner, E., Schrum, L.W., Schmidt, C.M. & McKillop, I.H. Rodent models of alcoholic liver disease: Of mice and men. Alcohol 46, 715–725 (2012).
Brandon-Warner, E., Walling, T.L., Schrum, L.W. & McKillop, I.H. Chronic ethanol feeding accelerates hepatocellular carcinoma progression in a sex-dependent manner in a mouse model of hepatocarcinogenesis. Alcohol. Clin. Exp. Res. 36, 641–653 (2012).
Gabele, E. et al. A new model of interactive effects of alcohol and high-fat diet on hepatic fibrosis. Alcohol. Clin. Exp. Res. 35, 1361–1367 (2011).
Wei, V.L. & Singh, S.M. Genetically determined response of hepatic aldehyde dehydrogenase activity to ethanol exposures may be associated with alcohol sensitivity in mouse genotypes. Alcohol. Clin. Exp. Res. 12, 39–45 (1988).
You, M., Considine, R.V., Leone, T.C., Kelly, D.P. & Crabb, D.W. Role of adiponectin in the protective action of dietary saturated fat against alcoholic fatty liver in mice. Hepatology 42, 568–577 (2005).
Everitt, H. et al. Ethanol administration exacerbates the abnormalities in hepatic lipid oxidation in genetically obese mice. Am. J. Physiol. Gastrointest. Liver Physiol. 304, G38–G47 (2013).
Tsuchiya, M. et al. Interstrain differences in liver injury and one-carbon metabolism in alcohol-fed mice. Hepatology 56, 130–139 (2012).
Butura, A. et al. The impact of CYP2E1 on the development of alcoholic liver disease as studied in a transgenic mouse model. J. Hepatol. 50, 572–583 (2009).
Lu, Y., Zhuge, J., Wang, X., Bai, J. & Cederbaum, A.I. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology 47, 1483–1494 (2008).
Kono, H. et al. Gender differences in early alcohol-induced liver injury: role of CD14, NF-κB, and TNF-α. Am. J. Physiol. Gastrointest. Liver Physiol. 278, G652–G661 (2000).
Nanji, A.A. et al. Increased severity of alcoholic liver injury in female rats: role of oxidative stress, endotoxin, and chemokines. Am. J. Physiol. Gastrointest. Liver Physiol. 281, G1348–G1356 (2001).
Kirpich, I.A. et al. The type of dietary fat modulates intestinal tight junction integrity, gut permeability, and hepatic toll-like receptor expression in a mouse model of alcoholic liver disease. Alcohol. Clin. Exp. Res. 36, 835–846 (2012).
Acknowledgements
We greatly appreciate all current and previous lab members for their technical support and helpful discussions. We also thank L. Chedester, A. Barnes and R. Kechrid from the Office of Laboratory Animal Science for their technical and engineering support, and for their critical reading of the manuscript. This work is supported by the intramural program of NIAAA at the NIH.
Author information
Authors and Affiliations
Contributions
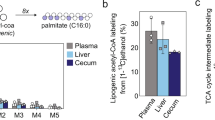
A.B. contributed to generating the data presented, making the figures and tables and drafting the procedures; S.M. contributed to drafting the materials and generating data presented in Figure 4; S.H.K. and H.W. contributed to generating the data presented in Figure 4 and B.G. contributed to drafting and the completion of the manuscript through supervision of others.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Bertola, A., Mathews, S., Ki, S. et al. Mouse model of chronic and binge ethanol feeding (the NIAAA model). Nat Protoc 8, 627–637 (2013). https://doi.org/10.1038/nprot.2013.032
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2013.032
This article is cited by
-
Akkermansia muciniphila Improves Depressive-Like Symptoms by Modulating the Level of 5-HT Neurotransmitters in the Gut and Brain of Mice
Molecular Neurobiology (2024)
-
RNA methylations in hepatic fibrosis, a gradually emerging new treatment strategy
Cell & Bioscience (2023)
-
A clinical experience-based Chinese herbal formula improves ethanol-induced drunken behavior and hepatic steatohepatitis in mice models
Chinese Medicine (2023)
-
Modulating phenylalanine metabolism by L. acidophilus alleviates alcohol-related liver disease through enhancing intestinal barrier function
Cell & Bioscience (2023)
-
Hepatocyte-derived MANF mitigates ethanol-induced liver steatosis in mice via enhancing ASS1 activity and activating AMPK pathway
Acta Pharmacologica Sinica (2023)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.